Abstract
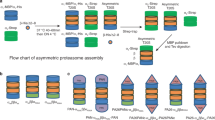
The 26S proteasome is a multisubunit protease responsible for regulated proteolysis in eukaryotic cells1,2. It comprises one catalytic 20S proteasome and two axially positioned 19S regulatory complexes3. The 20S proteasome is composed of 28 subunits arranged in a cylindrical particle as four heteroheptameric rings, α1–7β1–7β1–7α1–7 (refs 4, 5), but the mechanism responsible for the assembly of such a complex structure remains elusive. Here we report two chaperones, designated proteasome assembling chaperone-1 (PAC1) and PAC2, that are involved in the maturation of mammalian 20S proteasomes. PAC1 and PAC2 associate as heterodimers with proteasome precursors and are degraded after formation of the 20S proteasome is completed. Overexpression of PAC1 or PAC2 accelerates the formation of precursor proteasomes, whereas knockdown by short interfering RNA impairs it, resulting in poor maturation of 20S proteasomes. Furthermore, the PAC complex provides a scaffold for α-ring formation and keeps the α-rings competent for the subsequent formation of half-proteasomes. Thus, our results identify a mechanism for the correct assembly of 20S proteasomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pickart, C. M. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 70, 503–533 (2001)
Glickman, M. H. & Ciechanover, A. The ubiquitin–proteasome proteolytic pathway: destruction for the sake of construction. Physiol. Rev. 82, 373–428 (2002)
Baumeister, W., Walz, J., Zuhl, F. & Seemuller, E. The proteasome: paradigm of a self-compartmentalizing protease. Cell 92, 367–380 (1998)
Groll, M. et al. Structure of 20S proteasome from yeast at 2.4 Å resolution. Nature 386, 463–471 (1997)
Unno, M. et al. The structure of the mammalian 20S proteasome at 2.75 Å resolution. Structure (Camb.) 10, 609–618 (2002)
Zwickl, P., Kleinz, J. & Baumeister, W. Critical elements in proteasome assembly. Nat. Struct. Biol. 1, 765–770 (1994)
Gerards, W. L. et al. The human α-type proteasomal subunit HsC8 forms a double ringlike structure, but does not assemble into proteasome-like particles with the β-type subunits HsDelta or HsBPROS26. J. Biol. Chem. 272, 10080–10086 (1997)
Yao, Y. et al. α5 subunit in Trypanosoma brucei proteasome can self-assemble to form a cylinder of four stacked heptamer rings. Biochem. J. 344, 349–358 (1999)
Yang, Y., Fruh, K., Ahn, K. & Peterson, P. A. In vivo assembly of the proteasomal complexes, implications for antigen processing. J. Biol. Chem. 270, 27687–27694 (1995)
Chen, P. & Hochstrasser, M. Autocatalytic subunit processing couples active site formation in the 20S proteasome to completion of assembly. Cell 86, 961–972 (1996)
Schmidtke, G. et al. Analysis of mammalian 20S proteasome biogenesis: the maturation of β-subunits is an ordered two-step mechanism involving autocatalysis. EMBO J. 15, 6887–6898 (1996)
Nandi, D., Woodward, E., Ginsburg, D. B. & Monaco, J. J. Intermediates in the formation of mouse 20S proteasomes: implications for the assembly of precursor β subunits. EMBO J. 16, 5363–5375 (1997)
Schmidtke, G., Schmidt, M. & Kloetzel, P. M. Maturation of mammalian 20 S proteasome: purification and characterization of 13 S and 16 S proteasome precursor complexes. J. Mol. Biol. 268, 95–106 (1997)
Ramos, P. C., Hockendorff, J., Johnson, E. S., Varshavsky, A. & Dohmen, R. J. Ump1p is required for proper maturation of the 20S proteasome and becomes its substrate upon completion of the assembly. Cell 92, 489–499 (1998)
Griffin, T. A., Slack, J. P., McCluskey, T. S., Monaco, J. J. & Colbert, R. A. Identification of proteassemblin, a mammalian homologue of the yeast protein, Ump1p, that is required for normal proteasome assembly. Mol. Cell. Biol. Res. Commun. 3, 212–217 (2000)
Witt, E. et al. Characterisation of the newly identified human Ump1 homologue POMP and analysis of LMP7(β5i) incorporation into 20 S proteasomes. J. Mol. Biol. 301, 1–9 (2000)
Burri, L. et al. Identification and characterization of a mammalian protein interacting with 20S proteasome precursors. Proc. Natl Acad. Sci. USA 97, 10348–10353 (2000)
Natsume, T. et al. A direct nanoflow liquid chromatography-tandem mass spectrometry system for interaction proteomics. Anal. Chem. 74, 4725–4733 (2002)
Vidal-Taboada, J. M. et al. Down syndrome critical region gene 2: expression during mouse development and in human cell lines indicates a function related to cell proliferation. Biochem. Biophys. Res. Commun. 272, 156–163 (2000)
Bahar, R. et al. Growth retardation, polyploidy, and multinucleation induced by Clast3, a novel cell cycle-regulated protein. J. Biol. Chem. 277, 40012–40019 (2002)
Ahn, K. et al. In vivo characterization of the proteasome regulator PA28. J. Biol. Chem. 271, 18237–18242 (1996)
Tanahashi, N. et al. Hybrid proteasomes. Induction by interferon-γ and contribution to ATP-dependent proteolysis. J. Biol. Chem. 275, 14336–14445 (2000)
Murata, S. et al. Immunoproteasome assembly and antigen presentation in mice lacking both PA28α and PA28β. EMBO J. 20, 5898–5907 (2001)
Acknowledgements
We thank Y. Murakami for the ornithine decarboxylase degradation assay system, K. Furuyama for technical support, and D. Finley for comments on the manuscript. This work was supported by grants from the Japanese Science and Technology Agency (to S.M.), the Ministry of Education, Science and Culture of Japan (to S.M. and K.T.) and the New Energy and Industrial Technology Development Organization (to T.N.). Y.H. was supported by the Japanese Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The sequences for human PAC1 and PAC2 have been deposited in GenBank under accession numbers BR000236 and BR000237, respectively. Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
Supplementary Figures S1–S4. (PDF 2132 kb)
Supplementary Figure Legends
Text to accompany the above Supplementary Figures. (PDF 82 kb)
Supplementary Discussion
Additional discussion of results from the study. (PDF 127 kb)
Supplementary Methods
Description of additional methods used in this study. (PDF 81 kb)
Rights and permissions
About this article
Cite this article
Hirano, Y., Hendil, K., Yashiroda, H. et al. A heterodimeric complex that promotes the assembly of mammalian 20S proteasomes. Nature 437, 1381–1385 (2005). https://doi.org/10.1038/nature04106
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04106
This article is cited by
-
How to build a proteasome
Nature Structural & Molecular Biology (2021)
-
Heterozygous missense variant of the proteasome subunit β-type 9 causes neonatal-onset autoinflammation and immunodeficiency
Nature Communications (2021)
-
Complex-centric proteome profiling by SEC-SWATH-MS for the parallel detection of hundreds of protein complexes
Nature Protocols (2020)
-
Regulation of proteasome assembly and activity in health and disease
Nature Reviews Molecular Cell Biology (2018)
-
Structural insights on the dynamics of proteasome formation
Biophysical Reviews (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.