Abstract
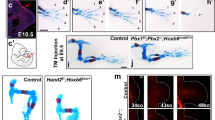
The vertebrate limb initially develops as a bud of mesenchymal cells that subsequently aggregate in a proximal to distal (P–D) sequence to give rise to cartilage condensations that prefigure all limb skeletal components1. Of the three cardinal limb axes, the mechanisms that lead to establishment and patterning of skeletal elements along the P–D axis are the least understood. Here we identify a genetic interaction between Gli3 (GLI-Kruppel family member 3) and Plzf (promyelocytic leukaemia zinc finger, also known as Zbtb16 and Zfp145), which is required specifically at very early stages of limb development for all proximal cartilage condensations in the hindlimb (femur, tibia, fibula). Notably, distal condensations comprising the foot are relatively unperturbed in Gli3-/-;Plzf-/- mouse embryos. We demonstrate that the cooperative activity of Gli3 and Plzf establishes the correct temporal and spatial distribution of chondrocyte progenitors in the proximal limb-bud independently of known P–D patterning markers and overall limb-bud size. Moreover, the limb defects in Gli3-/-;Plzf-/- embryos correlate with the transient death of a specific subset of proximal mesenchymal cells that express bone morphogenetic protein receptor, type 1B (Bmpr1b) at the onset of limb development. These findings suggest that the development of proximal and distal skeletal elements is distinctly regulated early during limb-bud formation. The initial division of the vertebrate limb into two distinct molecular domains is consistent with fossil evidence indicating that the upper and lower extremities of the limb have different evolutionary origins2.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hall, B. K. & Miyake, T. All for one and one for all: condensations and the initiation of skeletal development. Bioessays 22, 138–147 (2000)
Shubin, N., Tabin, C. & Carroll, S. Fossils, genes and the evolution of animal limbs. Nature 388, 639–648 (1997)
Wright, E. et al. The Sry-related gene Sox9 is expressed during chondrogenesis in mouse embryos. Nature Genet. 9, 15–20 (1995)
Akiyama, H., Chaboissier, M. C., Martin, J. F., Schedl, A. & de Crombrugghe, B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 16, 2813–2828 (2002)
Davis, A. P., Witte, D. P., Hsieh-Li, H. M., Potter, S. S. & Capecchi, M. R. Absence of radius and ulna in mice lacking hoxa-11 and hoxd-11. Nature 375, 791–795 (1995)
Boulet, A. M. & Capecchi, M. R. Multiple roles of Hoxa11 and Hoxd11 in the formation of the mammalian forelimb zeugopod. Development 131, 299–309 (2004)
Sun, X., Mariani, F. V. & Martin, G. R. Functions of FGF signalling from the apical ectodermal ridge in limb development. Nature 418, 501–508 (2002)
Barna, M., Hawe, N., Niswander, L. & Pandolfi, P. P. Plzf regulates limb and axial skeletal patterning. Nature Genet. 25, 166–172 (2000)
Barna, M. et al. Plzf mediates transcriptional repression of HoxD gene expression through chromatin remodeling. Dev. Cell 3, 499–510 (2002)
Hui, C. C. & Joyner, A. L. A mouse model of greig cephalopolysyndactyly syndrome: the extra-toesJ mutation contains an intragenic deletion of the Gli3 gene. Nature Genet. 3, 241–246 (1993)
Masuya, H., Sagai, T., Moriwaki, K. & Shiroishi, T. Multigenic control of the localization of the zone of polarizing activity in limb morphogenesis in the mouse. Dev. Biol. 182, 42–51 (1997)
Mercader, N. et al. Opposing RA and FGF signals control proximodistal vertebrate limb development through regulation of Meis genes. Development 127, 3961–3970 (2000)
Avantaggiato, V. et al. Developmental analysis of murine Promyelocyte Leukemia Zinc Finger (PLZF) gene expression: implications for the neuromeric model of the forebrain organization. J. Neurosci. 15, 4927–4942 (1995)
Yi, S. E., Daluiski, A., Pederson, R., Rosen, V. & Lyons, K. M. The type I BMP receptor BMPRIB is required for chondrogenesis in the mouse limb. Development 127, 621–630 (2000)
Pizette, S. & Niswander, L. BMPs are required at two steps of limb chondrogenesis: formation of prechondrogenic condensations and their differentiation into chondrocytes. Dev. Biol. 219, 237–249 (2000)
Zou, H., Wieser, R., Massague, J. & Niswander, L. Distinct roles of type I bone morphogenetic protein receptors in the formation and differentiation of cartilage. Genes Dev. 11, 2191–2203 (1997)
Wang, B., Fallon, J. F. & Beachy, P. A. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell 100, 423–434 (2000)
te Welscher, P. et al. Progression of vertebrate limb development through SHH-mediated counteraction of GLI3. Science 298, 827–830 (2002)
Litingtung, Y., Dahn, R. D., Y., Fallon, J. F. & Chiang, C. Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature 418, 979–983 (2002)
Oster, G., Shubin, N., Murray, J. & Alberch, P. Evolution and morphogenetic rules: the shape of the vertebrate limb in ontogeny and phylogeny. Evolution 42, 862–884 (1988)
Cohn, M. J., Lovejoy, C. O., Wolpert, L. & Coates, M. I. Branching, segmentation and the metapterygial axis: pattern versus process in the vertebrate limb. BioEssays 24, 460–465 (2002)
Wolpert, L., Tickle, C. & Sampford, M. The effect of cell killing by x–irradiation on pattern formation in the chick limb. J. Embryol. Exp. Morphol. 50, 175–193 (1979)
Summerbell, D. A. Quantitative analysis of the effect of excision of the AER from the chick limb-bud. J. Embryol. Exp. Morphol. 32, 651–660 (1974)
Dudley, A. T., Ros, M. A. & Tabin, C. J. A re-examination of proximodistal patterning during vertebrate limb development. Nature 418, 539–544 (2002)
Buscher, D., Bosse, B., Heymer, J. & Ruther, U. Evidence for genetic control of Sonic hedgehog by Gli3 in mouse limb development. Mech. Dev. 62, 175–182 (1997)
Wakamatsu, Y., Mochii, M., Vogel, K. S. & Weston, J. A. Avian neural crest-derived neurogenic precursors undergo apoptosis on the lateral migration pathway. Development 125, 4205–4213 (1998)
Acknowledgements
We thank our laboratory staff, in particular S. Weatherbee and I. Zohn, for discussions; D. Ruggero for suggestions and support; and Y. Yang for discussions on joint formation. We thank Y. Yang, C. Tabin, D. Kingsley, A. Boulet, M. Capecchi and B. de Crombrugghe for in situ hybridization probes. This work was supported by the NIH (L.N.) and by a MSKCC Cancer Center Support grant. L.N. is an Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figure S1
Limb skeletal phenotypes in mutant mice. (JPG 643 kb)
Supplementary Figure S2
Expression of joint and tendon markers in WT and Plzf-/-;Gli3-/- limbs. (JPG 313 kb)
Supplementary Figure S3
Histology of Plzf-/-;Gli3-/- hindlimb sections at E12.0 stained with hematoxylin and eosin. (JPG 679 kb)
Supplementary Figure S4
Expression of AER and A-P patterning genes in WT and Plzf-/-;Gli3-/- embryos. (JPG 524 kb)
Supplementary Figure S5
Expression of P-D patterning genes in Plzf-/-;Gli3-/- embryos. (JPG 535 kb)
Supplementary Figure S6
BMPR1B expression in the E10.5 limb. RNA in situ hybridization for BMPR1B on WT forelimb and hindlimb sections. (JPG 311 kb)
Rights and permissions
About this article
Cite this article
Barna, M., Pandolfi, P. & Niswander, L. Gli3 and Plzf cooperate in proximal limb patterning at early stages of limb development. Nature 436, 277–281 (2005). https://doi.org/10.1038/nature03801
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03801
This article is cited by
-
Promyelocytic leukemia zinc-finger induction signs mesenchymal stem cell commitment: identification of a key marker for stemness maintenance?
Stem Cell Research & Therapy (2014)
-
Dexamethasone-related osteogenic differentiation of dental follicle cells depends on ZBTB16 but not Runx2
Cell and Tissue Research (2014)
-
Time-Dependent Processes in Stem Cell-Based Tissue Engineering of Articular Cartilage
Stem Cell Reviews and Reports (2012)
-
Human intronic enhancers control distinct sub-domains of Gli3 expression during mouse CNS and limb development
BMC Developmental Biology (2010)
-
Tibiotalaire autologe kraakbe encelimplantatie: fundamentele aspecten van een biopsielocatie in de enkel
PodoSophia (2010)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.