Abstract
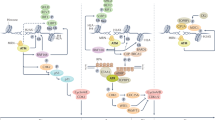
BRCA1 and BRCA2 are important for DNA double-strand break repair by homologous recombination1, and mutations in these genes predispose to breast and other cancers2. Poly(ADP-ribose) polymerase (PARP) is an enzyme involved in base excision repair, a key pathway in the repair of DNA single-strand breaks3. We show here that BRCA1 or BRCA2 dysfunction unexpectedly and profoundly sensitizes cells to the inhibition of PARP enzymatic activity, resulting in chromosomal instability, cell cycle arrest and subsequent apoptosis. This seems to be because the inhibition of PARP leads to the persistence of DNA lesions normally repaired by homologous recombination. These results illustrate how different pathways cooperate to repair damage, and suggest that the targeted inhibition of particular DNA repair pathways may allow the design of specific and less toxic therapies for cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tutt, A. & Ashworth, A. The relationship between the roles of BRCA genes in DNA repair and cancer predisposition. Trends Mol. Med. 8, 571–576 (2002)
Wooster, R. & Weber, B. Breast and ovarian cancer. N. Engl. J. Med. 348, 2339–2347 (2003)
Hoeijmakers, J. H. Genome maintenance mechanisms for preventing cancer. Nature 411, 366–374 (2001)
Schultz, N., Lopez, E., Saleh-Gohari, N. & Helleday, T. Poly(ADP-ribose) polymerase (PARP-1) has a controlling role in homologous recombination. Nucleic Acids Res. 31, 4959–4964 (2003)
Moynahan, M. E., Pierce, A. J. & Jasin, M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol. Cell 7, 263–272 (2001)
Moynahan, M. E., Chiu, J. W., Koller, B. H. & Jasin, M. Brca1 controls homology-directed DNA repair. Mol. Cell 4, 511–518 (1999)
Tutt, A. et al. Mutation in Brca2 stimulates error-prone homology-directed repair of DNA double-strand breaks occurring between repeated sequences. EMBO J. 20, 4704–4716 (2001)
Foray, N. et al. A subset of ATM- and ATR-dependent phosphorylation events requires the BRCA1 protein. EMBO J. 22, 2860–2871 (2003)
Kraakman-van der Zwet, M. et al. Brca2 (XRCC11) deficiency results in radioresistant DNA synthesis and a higher frequency of spontaneous deletions. Mol. Cell. Biol. 22, 669–679 (2002)
Furuta, T. et al. Phosphorylation of histone H2AX and activation of Mre11, Rad50, and Nbs1 in response to replication-dependent DNA double-strand breaks induced by mammalian DNA topoisomerase I cleavage complexes. J. Biol. Chem. 278, 20303–20312 (2003)
Bhattacharyya, A., Ear, U. S., Koller, B. H., Weichselbaum, R. R. & Bishop, D. K. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J. Biol. Chem. 275, 23899–23903 (2000)
Dantzer, F., et al., Involvement of poly(ADP-ribose) polymerase in base excision repair. Biochimie 81, 69–75 (1999)
Boulton, S., Kyle, S., & Durkacz, B. W. Interactive effects of inhibitors of poly(ADP-ribose) polymerase and DNA-dependent protein kinase on cellular to DNA damage. Carcinogenesis 20, 199–203 (1999)
Haber, J. E. DNA recombination: the replication connection. Trends Biochem. Sci. 24, 271–275 (1999)
Arnaudeau, C., Lundin, C. & Helleday, T. DNA double-strand breaks associated with replication forks are predominantly repaired by homologous recombination involving an exchange mechanism in mammalian cells. J. Mol. Biol. 307, 1235–1245 (2001)
Lomonosov, M., Anand, S., Sangrithi, M., Davies, R. & Venkitaraman, A. R. Stabilization of stalled DNA replication forks by the BRCA2 breast cancer susceptibility protein. Genes Dev. 17, 3017–3022 (2003)
Bryant, H. E. et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature doi:10.1038/nature03443 (this issue)
Wang, Z. Q. et al. PARP is important for genomic stability but dispensable in apoptosis. Genes Dev. 11, 2347–2358 (1997)
Turner, N., Tutt, A. & Ashworth, A. Hallmarks of BRCAness in sporadic cancers. Nature Rev. Cancer 4, 814–819 (2004)
Loh, V. M. et al. Phthalazinones. Part 1: The design and synthesis of a novel series of potent inhibitors of poly(ADP-ribose) polymerase. Bioinorg. Med. Chem. Lett. (in the press)
Ame, J.-C. et al. PARP-2, a novel mammalian DNA damage-dependent poly(ADP-ribose) polymerase. J. Biol. Chem. 274, 17860–17868 (1999)
Kickhoefer, V. A. et al. The 193-kD vault protein, VPARP, is a novel poly(ADP-ribose) polymerase. J. Cell Biol. 146, 917–928 (1999)
Dillon, K. J., Smith, G. C. M. & Martin, N. M. B. A flashplate assay for the identification of PARP-1 inhibitors. J. Biomol. Screen. 8, 347–352 (2003)
Acknowledgements
We thank Cancer Research UK, Breakthrough Breast Cancer and the Mary-Jean Mitchell Green Foundation for financial support. We thank I. Titley for help with FACS analysis, A. McCarthy and J. Williamson for help with chromosome spreads, E. Witt for western blot analysis, E. Iorns for real-time PCR analysis and M. Zdzienicka for V-C8 and V-C8 BAC cells. We also thank the Maybridge Chemical Company for their help in the design and synthesis of the PARP inhibitors.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
G.C.M.S., N.M.B.M., I.H., C.K. and K.J.D. are employees of KuDOS Pharmaceuticals Ltd. S.P.J. is the scientific founder and Chief Scientific Officer of KuDOS Pharmaceuticals Ltd. A patent application has been submitted by KuDOS and the Institute of Cancer Research based on these results.
Supplementary information
Supplementary Figures S1-S4
Supplementary Figure S1 describes the novel PARP inhibitor compounds used in this study. Supplementary Figure S2 shows a clonogenic experiment illustrating the rapid and irreversible effects of PARP inhibitors. Supplementary Figure S3 describes clonogenic experiments using Chinese Hamster Ovary and MCF7 cells and PARP inhibitors. Supplementary Figure S4 shows metaphase spreads, γH2AX assays and Rad51 assays. (PPT 1258 kb)
Supplementary Data
This file contains Supplementary Table S1, Supplementary Methods, Supplementary Figure Legends and additional references. (RTF 41 kb)
Rights and permissions
About this article
Cite this article
Farmer, H., McCabe, N., Lord, C. et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434, 917–921 (2005). https://doi.org/10.1038/nature03445
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03445
This article is cited by
-
Comprehensive machine learning boosts structure-based virtual screening for PARP1 inhibitors
Journal of Cheminformatics (2024)
-
PARP inhibitor era in ovarian cancer treatment: a systematic review and meta-analysis of randomized controlled trials
Journal of Ovarian Research (2024)
-
PSIP1/LEDGF reduces R-loops at transcription sites to maintain genome integrity
Nature Communications (2024)
-
UFL1 triggers replication fork degradation by MRE11 in BRCA1/2-deficient cells
Nature Chemical Biology (2024)
-
Transcription–replication conflicts underlie sensitivity to PARP inhibitors
Nature (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.