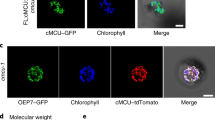
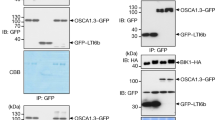
Abstract
Cytosolic free calcium ([Ca2+]cyt) is a ubiquitous signalling component in plant cells1. Numerous stimuli trigger sustained or transient elevations of [Ca2+]cyt that evoke downstream stimulus-specific responses. Generation of [Ca2+]cyt signals is effected through stimulus-induced opening of Ca2+-permeable ion channels that catalyse a flux of Ca2+ into the cytosol from extracellular or intracellular stores. Many classes of Ca2+ current have been characterized electrophysiologically in plant membranes2. However, the identity of the ion channels that underlie these currents has until now remained obscure. Here we show that the TPC1 (‘two-pore channel 1’) gene of Arabidopsis thaliana encodes a class of Ca2+-dependent Ca2+-release channel that is known from numerous electrophysiological studies as the slow vacuolar channel3,4,5. Slow vacuolar channels are ubiquitous in plant vacuoles, where they form the dominant conductance at micromolar [Ca2+]cyt. We show that a tpc1 knockout mutant lacks functional slow vacuolar channel activity and is defective in both abscisic acid-induced repression of germination and in the response of stomata to extracellular calcium. These studies unequivocally demonstrate a critical role of intracellular Ca2+-release channels in the physiological processes of plants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sanders, D., Pelloux, J., Brownlee, C. & Harper, J. F. Calcium at the crossroads of signaling. Plant Cell 14, S401–S417 (2002)
White, P. J. Calcium channels in higher plants. Biochim. Biophys. Acta 1465, 171–189 (2000)
Allen, G. J. & Sanders, D. Vacuolar ion channels of higher plants. Adv. Bot. Res. 25, 217–252 (1997)
Ward, J. M. & Schroeder, J. I. Calcium-activated K+ channels and calcium-induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. Plant Cell 6, 669–683 (1994)
Hedrich, R. & Neher, E. Cytoplasmic calcium regulates voltage-dependent ion channels in plant vacuoles. Nature 329, 833–836 (1987)
Furuichi, T., Cunningham, K. W. & Muto, S. A putative two-pore channel AtTPC1 mediates Ca2+ flux in Arabidopsis leaf cells. Plant Cell Physiol. 42, 900–905 (2001)
Kadota, Y. et al. Identification of putative voltage-dependent Ca2+-permeable channels involved in cryptogein-induced Ca2+ transients and defense responses in tobacco BY-2 cells. Biochem. Biophys. Res. Commun. 317, 823–830 (2004)
Hashimoto, K., Saito, M., Matsuoka, H., Iida, K. & Iida, H. Functional analysis of a rice putative voltage-dependent Ca2+ channel, OsTPC1, expressed in yeast cells lacking its homologous gene CCH1. Plant Cell Physiol. 45, 496–500 (2004)
Kurusu, T., Sakurai, Y., Miyao, A., Hirochika, H. & Kuchitsu, K. Identification of a putative voltage-gated Ca2+-permeable channel (OsTPC1) involved in Ca2+ influx and regulation of growth and development in rice. Plant Cell Physiol. 45, 693–702 (2004)
Ishibashi, K., Suzuki, M. & Imai, M. Molecular cloning of a novel form (two-repeat) protein related to voltage-gated sodium and calcium channels. Biochem. Biophys. Res. Commun. 270, 370–376 (2000)
Szponarski, W., Sommerer, N., Boyer, J-C., Rossignol, M. & Gibrat, R. Large-scale characterization of integral proteins from Arabidopsis vacuolar membrane by two-dimensional liquid chromatography. Proteomics 4, 397–406 (2004)
Carter, C. et al. The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins. Plant Cell 16, 3285–3303 (2004)
Schönknecht, G. et al. KCO1 is a component of the slow-vacuolar (SV) ion channel. FEBS Lett. 511, 28–32 (2002)
Bewell, M. A., Maathuis, F. J. M., Allen, G. J. & Sanders, D. Calcium-induced calcium release mediated by a voltage-activated cation channel in vacuolar vesicles from red beet. FEBS Lett. 458, 41–44 (1999)
Pottosin, I. I., Dobrovinskaya, O. R. & Muniz, J. Conduction of monovalent and divalent cations in the slow vacuolar channel. J. Membr. Biol. 181, 55–65 (2001)
Allen, G. J. & Sanders, D. Control of ionic currents in guard cell vacuoles by cytosolic and lumenal calcium. Plant J. 10, 1055–1069 (1996)
Leonhardt, N. et al. Microarray expression analyses of Arabidopsis guard cells and isolation of a recessive abscisic acid hypersensitive protein phosphatase 2C mutant. Plant Cell 16, 596–615 (2004)
Finkelstein, R. R., Gampala, S. S. L. & Rock, C. D. Abscisic acid signaling in seeds and seedlings. Plant Cell 14, S15–S45 (2002)
Schroeder, J. I., Allen, G. J., Hugouvieux, V., Kwak, J. M. & Waner, D. Guard cell signal transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 627–658 (2001)
McAinsh, M. R., Webb, A. A. R., Taylor, J. E. & Hetherington, A. M. Stimulus-induced oscillations in guard cell cytoplasmic free calcium. Plant Cell 7, 1207–1219 (1995)
Hetherington, A. M. & Woodward, F. I. The role of stomata in sensing and driving environmental change. Nature 424, 901–908 (2003)
Allen, G. J. et al. Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289, 2338–2342 (2000)
Alonso, J. M. et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301, 653–657 (2003)
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998)
Gleave, A. P. A versatile binary vector system with a T-DNA organisational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol. Biol. 20, 1203–1207 (1992)
Abel, S. & Theologis, A. Transient transformation of Arabiodopsis leaf protoplasts: a versatile experimental system to study gene expression. Plant J. 5, 421–427 (1994)
Maathuis, F. J. M. & Sanders, D. Patch-clamping plant cells. Methods Cell Biol. 49, 293–302 (1995)
Maathuis, F. J. M. & Sanders, D. Sodium uptake in Arabidopsis roots is regulated by cyclic nucleotides. Plant Physiol. 127, 1617–1625 (2001)
Muir, S. R. & Sanders, D. Inositol 1,4,5-triphosphate-sensitive Ca2+ release across nonvacuolar membranes in cauliflower. Plant Physiol. 114, 1511–1521 (1997)
Höfte, H., Faye, L., Dickinson, C., Herman, E. M. & Chrispeels, M. J. The protein-body proteins phytohemagglutinin and tonoplast intrinsic protein are targeted to vacuoles in leaves of transgenic tobacco. Planta 184, 431–437 (1991)
Acknowledgements
We thank M. Fischer and R. V. Jolley for initial experiments, L. Skiera and S. Kilmartin for technical assistance, P. O'Toole for advice on confocal microscopy, G. Park for preparing the pART7GFP construct, M. G. Palmgren for providing AHA2 antibodies, and J. I. Schroeder for discussions. This work was funded by the Biotechnology and Biological Sciences Research Council and an award by the Koerber Foundation to D.S.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Peiter, E., Maathuis, F., Mills, L. et al. The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature 434, 404–408 (2005). https://doi.org/10.1038/nature03381
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03381
This article is cited by
-
Linking high light-induced cellular ionic and oxidative responses in leaves to fruit quality in tomato
Plant Growth Regulation (2023)
-
Comparative analysis of stress-induced calcium signals in the crop species barley and the model plant Arabidopsis thaliana
BMC Plant Biology (2022)
-
Electrophysiology and fluorescence to investigate cation channels and transporters in isolated plant vacuoles
Stress Biology (2022)
-
Reducing potassium deficiency by using sodium fertilisation
Stress Biology (2022)
-
Major vacuolar TPC1 channel in stress signaling: what matters, K+, Ca2+ conductance or an ion-flux independent mechanism?
Stress Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.