Abstract
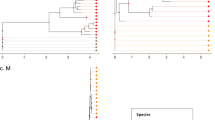
Although parasite–host co-speciation is a long-held hypothesis, convincing evidence for long-term co-speciation remains elusive, largely because of small numbers of hosts and parasites studied and uncertainty over rates of evolutionary change1,2,3,4,5. Co-speciation is especially rare in RNA viruses, in which cross-species transfer is the dominant mode of evolution6,7,8,9. Simian foamy viruses (SFVs) are ubiquitous, non-pathogenic retroviruses that infect all primates10,11. Here we test the co-speciation hypothesis in SFVs and their primate hosts by comparing the phylogenies of SFV polymerase and mitochondrial cytochrome oxidase subunit II from African and Asian monkeys and apes. The phylogenetic trees were remarkably congruent in both branching order and divergence times, strongly supporting co-speciation. Molecular clock calibrations revealed an extremely low rate of SFV evolution, 1.7 × 10-8 substitutions per site per year, making it the slowest-evolving RNA virus documented so far. These results indicate that SFVs might have co-speciated with Old World primates for at least 30 million years, making them the oldest known vertebrate RNA viruses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hafner, M. S. et al. Disparate rates of molecular evolution in cospeciating hosts and parasites. Science 265, 1087–1090 (1994)
Page, R. D. M. Temporal congruence and cladistic analysis of biogeography and cospeciation. Syst. Zool. 39, 205–226 (1990)
Brooks, D. R. Analysis of host-parasite coevolution. Int. J. Parasitol. 17, 291–297 (1987)
Charrel, R. N., De Micco, P. & de Lamballerie, X. Phylogenetic analysis of GB viruses A and C: evidence for cospeciation between virus isolates and their primate hosts. J. Gen. Virol. 80, 2329–2335 (1999)
McGeoch, D. J., Dolan, A. & Ralph, A. C. Toward a comprehensive phylogeny for mammalian and avian herpesviruses. J. Virol. 74, 10401–10406 (2000)
Charleston, M. A. & Robertson, D. L. Preferential host switching by primate lentiviruses can account for phylogenetic similarity with the primate phylogeny. Syst. Biol. 51, 528–535 (2002)
Salemi, M. et al. Mosaic genomes of the six major primate lentivirus lineages revealed by phylogenetic analyses. J. Virol. 77, 7202–7213 (2003)
Jackson, A. P. & Charleston, M. A. A cophylogenetic perspective of RNA-virus evolution. Mol. Biol. Evol. 21, 45–57 (2004)
Holmes, E. C. Molecular clocks and the puzzle of RNA virus origins. J. Virol. 77, 3893–3897 (2003)
Meiering, C. D. & Linial, M. L. Historical perspective of foamy virus epidemiology and infection. Clin. Microbiol. Rev. 14, 165–176 (2001)
Hussain, A. I. et al. Screening for simian foamy virus infection by using a combined antigen Western blot assay: evidence for a wide distribution among Old World primates and identification of four new divergent viruses. Virology 309, 248–257 (2003)
Disotell, T. R., Honeycutt, R. L. & Ruvolo, M. Mitochondrial DNA phylogeny of the Old-World monkey tribe Papionini. Mol. Biol. Evol. 9, 1–13 (1992)
Ruvolo, M., Disotell, T. R., Allard, M. W., Brown, W. M. & Honeycutt, R. L. Resolution of the African hominoid trichotomy by use of a mitochondrial gene sequence. Proc. Natl Acad. Sci. USA 88, 1570–1574 (1991)
Adkins, R. M. & Honeycutt, R. L. Evolution of the primate cytochrome C oxidase subunit II gene. J. Mol. Evol. 38, 215–231 (1994)
van der Kuyl, A. C., Kuiken, C. L., Dekker, J. T. & Goudsmit, J. Phylogeny of African monkeys based upon mitochondrial 12S rRNA sequences. J. Mol. Evol. 40, 173–180 (1995)
Page, S. L., Chiu, C. H. & Goodman, M. Molecular phylogeny of Old World monkeys (Cercopithecidae) as inferred from gamma-globin DNA sequences. Mol. Phylogenet. Evol. 13, 348–359 (1999)
Harris, E. E. & Disotell, T. R. Nuclear gene trees and the phylogenetic relationships of the mangabeys (Primates: Papionini). Mol. Biol. Evol. 15, 892–900 (1998)
Goodman, M. et al. Toward a phylogenetic classification of Primates based on DNA evidence complemented by fossil evidence. Mol. Phylogenet. Evol. 9, 585–598 (1998)
Page, S. L. & Goodman, M. Catarrhine phylogeny: noncoding DNA evidence for a diphyletic origin of the mangabeys and for a human-chimpanzee clade. Mol. Phylogenet. Evol. 18, 14–25 (2001)
Murphy, W. J. et al. Resolution of the early placental mammal radiation using Bayesian phylogenetics. Science 294, 2348–2351 (2001)
Sanderson, M. J. r8s: inferring absolute rates of molecular evolution and divergence times in the absence of a molecular clock. Bioinformatics 19, 301–302 (2003)
Ruvolo, M. et al. Mitochondrial COII sequences and modern human origins. Mol. Biol. Evol. 10, 1115–1135 (1993)
Shadan, F. F. & Villarreal, L. P. Coevolution of persistently infecting small DNA viruses and their hosts linked to host-interactive regulatory domains. Proc. Natl Acad. Sci. USA 90, 4117–4121 (1993)
Johnson, W. E. & Coffin, J. M. Constructing primate phylogenies from ancient retrovirus sequences. Proc. Natl Acad. Sci. USA 96, 10254–10260 (1999)
Suzuki, Y., Yamaguchi-Kabata, Y. & Gojobori, T. Nucleotide substitution rates of HIV-1. AIDS Rev. 2, 39–47 (2000)
Löchelt, M. Foamy virus transactivation and gene expression. Curr. Top. Microbiol. Immunol. 277, 27–61 (2003)
Woelk, C. H. & Holmes, E. C. Reduced positive selection in vector-borne RNA viruses. Mol. Biol. Evol. 19, 2333–2336 (2002)
Groves, C. Primate Taxonomy (Smithsonian Institution Press, Washington DC, 2001)
Sanderson, M. J. Estimate absolute rates of molecular evolution and divergence times: a penalized likelihood approach. Mol. Biol. Evol. 19, 101–109 (2002)
Page, R. D. M. Parallel phylogenies: reconstructing the history of host-parasite assemblages. Cladistics 10, 155–173 (1994)
Acknowledgements
We thank the veterinary staff at all zoological gardens and primate centres who kindly provided blood specimens from the primates living at their institutions; R. Heberle and P. Johnston for the SFV-infected baboon and orangutan isolates; A. Hussain and A. Wright for expert technical assistance; and A. Vandamme, C. Coulibaly, M. Peeters, F. Bibollet-Ruche, V. Hirsch, J. Allan, T. Butler and H. McClure for providing additional primate samples for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Tables 1 and 2
This file includes a list of the specimens used in this study and the corresponding taxon codes for the primate and SFV sequences shown in Figs 1-3. (PDF 39 kb)
Rights and permissions
About this article
Cite this article
Switzer, W., Salemi, M., Shanmugam, V. et al. Ancient co-speciation of simian foamy viruses and primates. Nature 434, 376–380 (2005). https://doi.org/10.1038/nature03341
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03341
This article is cited by
-
Co-structure analysis and genetic associations reveal insights into pinworms (Trypanoxyuris) and primates (Alouatta palliata) microevolutionary dynamics
BMC Ecology and Evolution (2021)
-
Evolutionary stasis of viruses?
Nature Reviews Microbiology (2019)
-
Foamy virus zoonotic infections
Retrovirology (2017)
-
Marine origin of retroviruses in the early Palaeozoic Era
Nature Communications (2017)
-
Divergent viral presentation among human tumors and adjacent normal tissues
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.