Abstract
Concern about biological weapons has raised questions about the most effective public health policies to contain an anthrax outbreak1,2,3. We developed a probability model to predict the impact of different anthrax antibiotic and vaccination policies. An anthrax outbreak can be significantly contained by minimizing the delay until initiation of antibiotic prophylaxis. However, even if mass distribution of antibiotics is completed within six days of the initial exposure, then at most about 70% of cases can be prevented. Post-exposure vaccination will not significantly increase that prevention rate if adherence to antibiotic regimens is similar or higher than that attained in the 2001 US outbreak4. However, post-exposure vaccination can be useful either in shortening the duration of a prolonged antibiotic regimen, in the event of an antibiotic-resistant strain, or if antibiotic adherence rates are very low. Here we show that a mass pre-exposure vaccination programme for the general population would require very high population coverage rates to significantly increase prevention rates from that achieved with targeted and rapid post-exposure prophylaxis programmes.
Similar content being viewed by others
Main
Public health planners are uncertain how an anthrax vaccine should be used as part of a comprehensive strategy for addressing the bioterrorism threats posed by Bacillus anthracis1,2,3. Critical public health questions include whether an anthrax vaccine should be used before exposure to immunize the general population, or used as a post-exposure prophylaxis. The answers depend on many factors including the safety and efficacy of prolonged antibiotic prophylaxis, adherence to antibiotic regimens, the time delay before post-exposure antibiotic prophylaxis is initiated and vaccine characteristics such as efficacy, safety and time to achieve immunity. The US Food and Drug administration licensed an anthrax vaccine, Anthrax Vaccine Absorbed (AVA) in 1970. Because AVA can require six doses and up to 18 months to achieve maximum protection, research has accelerated to develop and manufacture an improved anthrax vaccine. It is hoped that recombinant protective antigen (rPA) anthrax vaccine could provide maximum protection in three doses over a period of not more than 28 days2,5,6.
The Centers for Disease Control and Prevention recommended that those people exposed in the 2001 US anthrax attacks receive 60 days of antibiotic prophylaxis. Subsequently, it was recommended that people consider extending the antibiotic therapy with or without three doses of the anthrax vaccine3,7. However, few opted to take this additional recommendation, and adherence to the initially recommended 60-day antibiotic regimen was less than 50%8. Nevertheless, no persons among the more than 10,000 recommended for prophylaxis therapy developed disease and among them only a handful of cases were prevented9. This experience has raised questions about which public health strategies most effectively contain an anthrax outbreak.
A 1-kg release of an anthrax preparation could contain more than 1014 spores, roughly equivalent to five-billion lethal doses10,11. Although the magnitude of such a release seems daunting, the critical determinants for public health involve not just the size of the release but also dispersal factors, which include the size of the spores, the type of spore preparation (wet or dry), method of release, wind conditions, temperatures, building insulation and the population density of the exposed areas (both inside and outside of buildings)2,10,11,12. Atmospheric models predict that a 100-kg release could result in between tens of thousands to several million deaths, depending on these factors2,11. In the face of this uncertainty, how does one determine rational public health policy?
Quantitative models have been used recently to inform the policy debate about smallpox vaccination strategies13,14,15,16,17. To evaluate strategies for containing an anthrax outbreak, we developed a probability model that accounts for the dynamics of spore clearance and germination, and describes the effects of pre-exposure vaccination, post-exposure vaccination, antibiotic prophylaxis and antibiotic adherence. We present the results in terms of the percentages of cases that can be prevented with various public health policies. We show that these percentages do not vary appreciably by exposure level (dose of inhaled spores), thus allowing public health policy to be guided, even with the great uncertainty surrounding the specifics of bioterrorism threats.
We considered three models for the dose of inhaled spores: a fixed high-exposure level in which all persons are exposed to the infectious dose ID(50), which yields a rate of 5,000 cases per 10,000 exposed persons with no public health intervention; a fixed low-exposure level in which all persons are exposed to the ID(1) and which yields a rate of 100 cases per 10,000 exposed; and a variable moderate exposure level in which the numbers of inhaled spores varied across the population and yields an average rate of 1,020 cases per 10,000 exposed.
Table 1 displays the percentage of cases prevented with various post-exposure interventions. Surprisingly, the results do not vary appreciably by exposure level. For example, with a rapid 6-day response in which mass antibiotic distribution begins 3 days after the initial exposure and is completed after an additional 3 days, the percentage of cases prevented with full 60-day antibiotic adherence were 68%, 67% and 75% for the high, variable moderate and low exposures, respectively. With a slower 12-day response in which mass antibiotic distribution begins 6 days after the initial exposure and is completed after an additional 6 days, the corresponding percentages of cases prevented were 46%, 46% and 55%. Figure 1 shows the relationship between the percentage of cases prevented and the delay until beginning mass antibiotic distribution. The figure shows that prevention rates, which do not vary significantly by exposure level, are high with short delays, but fall to under 50% after a 10-day delay.
We considered the situation when there was only partial adherence to the antibiotic regimen; specifically, when 25% of people completed either 15, 30, 45 or 60 days of a 60-day regimen, similar to that observed in the 2001 US anthrax outbreak8. The percentages of cases prevented drops by about 7–10% compared with full antibiotic adherence (Table 1).
We evaluated the preventive value of post-exposure vaccination as an adjuvant to antibiotic prophylaxis. Our first set of calculations assumed a 95% vaccine efficacy with a 28-day delay to achieve vaccine-induced immunity5. If people adhere to the antibiotic regimen, then addition of post-exposure vaccination increases the per cent prevented by, at most, 1% (Table 1). If people only partially adhere to the antibiotic regimen, post-exposure vaccination modestly increases the prevention rate by no more than 6% (Table 1). If the strain of B. anthracis is resistant to antibiotics, then post-exposure vaccination prevents between 6% and 9% of cases depending on the exposure level (row 1, Table 1). These modest preventive effects of post-exposure vaccination are explained by the time delay to achieve vaccine-induced immunity.
We performed a sensitivity analysis to the time delay to achieve vaccine-induced immunity, to identify characteristics that would improve a vaccine's utility as a post-exposure prophylaxis. Table 2 shows the percentages of cases prevented under different assumptions of vaccine immunogenicity. For example, we considered a highly immunogenic vaccine in which 25%, 50%, 75% and 95% of people achieve immunity within 7, 14, 21 and 28 days following the first vaccine dose, respectively (last row, Table 2). We find that the addition of such a vaccine to an effective antibiotic regimen results in an increase in the prevention rate of no more than 9% in all situations considered (including one in which antibiotics were only partially (90%) effective). A vaccine that also reduced disease severity could have an additional benefit of increasing the percentages of deaths prevented. For example, suppose people are exposed to high doses of an antibiotic-resistant strain; if a single dose of a highly immunogenic vaccine given before disease onset also reduced the mortality rate among cases by 50%, 25%, 10% and 0%, then the percentages of deaths prevented are 25%, 21%, 18% and 17%, respectively.
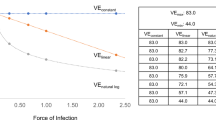
Prolonged antibiotic prophylaxis may be indicated if either exposure levels to spores are high, or there is significant risk of re-exposure from either re-aerosolization of spores during remediation of contaminated areas or from another bioterrorist event. Shortening a long course of antibiotics may be critical because of difficulty in maintaining adherence, limited supplies of antibiotics, resistance and adverse events associated with long-term antibiotic therapy. We examined the trade-offs associated with shortening the antibiotic regimen by replacing antibiotic protection with post-exposure vaccination. Vaccination confers long-term immunity, whereas antibiotics are effective for only as long as they are being taken. However, that advantage of vaccination must be balanced by the fact that vaccine efficacy is not 100%. We calculated how many days an antibiotic regimen could be shortened by post-exposure vaccination while not increasing total disease risk, assuming antibiotic efficacy was 100%. The results depend on the vaccine efficacy (Fig. 2). For example, if the vaccine efficacy is 95%, 90% and 80%, then antibiotic regimens could be shortened 43, 33 and 23 days, respectively, provided that the level of vaccine efficacy is achieved before antibiotics are terminated. For example, a 120-day antibiotic regimen could be shortened to only 77 days by using a vaccine that was 95% efficacious.
Table 1 suggests that post-exposure antibiotics can prevent at most about 70% of cases. We calculated whether a mass pre-exposure vaccination programme of the general population could significantly increase that prevention rate. Table 3 shows levels of pre-exposure vaccine coverage in the general population that would be necessary to increase the percentages of cases prevented to either 75% or 90%. The results show that the required level of vaccine coverage depends on multiple factors including exposure level, antibiotic response time and adherence; nevertheless, some general conclusions are possible. To prevent 90% of cases, at the very least 63% of the population would need to receive pre-exposure vaccination. That minimal level of vaccine coverage applies when the exposure is low with rapid response and full antibiotic adherence. Generally, vaccine coverage levels that are considerably higher than 63% are required when the response is slower and antibiotic adherence is lower (Table 3).
Our analysis shows that minimizing the delay until initiation of antibiotic prophylaxis is key to containing an anthrax outbreak, and reinforces the results of Wein et al.11. Heightened clinical awareness of symptoms, improved public health surveillance, and efficient systems for mass antibiotic distribution11,18,19 are all critical components in reducing response time. In the 2001 US outbreak, postal workers did not receive antibiotics until more than 9 days after exposure. However, even if mass antibiotic distribution is completed within 6 days of exposure, at most about 70% of cases could be prevented. Our analysis shows that post-exposure vaccination should not be expected to significantly increase that percentage because of the delays to achieve vaccine protection, but nevertheless can be useful in shortening a prolonged antibiotic regimen. Although mass pre-exposure vaccination programmes of the general population do increase prevention rates, very high vaccine coverage rates are required.
These analyses assumed that there are adequate supplies of antibiotics and vaccine, and that all exposed persons needing post-exposure prophylaxis are completely identified. However, if only a fraction, γ, of exposed persons are identified, then the prevention rates in Table 1 would be overestimated and should then be multiplied by γ. In the event of a large bioterrorism attack, γ could well be less than 1. The analyses assumed that implementation of post-exposure response policies, including identification of exposed persons, does not depend on the numbers of exposed persons. Wein and colleagues11 considered models that account for the attack size and the queues that would result from limited resources for an emergency response. Developing bioterrorism response policies is challenging because of limited available data on anthrax outbreaks. Although we cannot plan for all possible events of bioterrorism, having public health policy guidelines in place for rapid and rational decision-making are critical elements of public health preparedness.
Methods
A probability model based on a competing risks formulation of spore clearance and germination20 was developed to describe the impact of disease control strategies. The risk that a spore germinates is called λ, and the risk that a spore is naturally cleared from the lung by natural mechanisms—including being expelled through a bronchus, swallowed or killed by macrophages—is called the clearance rate θ. These risks are the hazard rates, expressed in units of per day. In addition to these natural clearance mechanisms, a person can be protected from disease by antibiotics or vaccine. If antibiotics are circulating in the body at the time a spore begins to germinate, or if the person has been vaccinated, then it is assumed that the germinating spore is destroyed with probabilities determined by the efficacies of the antibiotics or vaccine. If a spore successfully evades the natural clearance mechanisms, germinates and is not destroyed by vaccine-induced immunity or antibiotics, then it multiplies rapidly, and produces toxin and disease21,22,23,24,25.
Impact of post-exposure prophylaxis
Suppose protection from disease begins at time t1 then stops at t2, and perhaps begins again at t3, where the times are measured in days from exposure. Intermittent protection from disease could result from starting and stopping antibiotics, and achieving vaccine-induced immunity.
The probability R1 that a spore germinates in the interval (0, t1) is given by:

The probability R2 that a spore germinates in the interval (t1, t2) is equal to 0. The probability R3 that a spore germinates in the interval (t2, t3) is given by:

The probability R4 that a spore germinates after t3 is equal to 0.
Suppose a person inhales D spores, and let X be the number of spores that will ever germinate in that individual. Assuming X has a Poisson distribution with mean µ = D (R1 + R2 + R3 + R4), then the cumulative probability that at least one spore germinates is c = 1 - e-µ, which is:

The estimates of θ from animal data are approximately 0.07 per day, that is, spores are cleared from the lung at about 7% per day20,26. That estimate of the clearance rate is consistent with human data on the incubation period from the largest documented human anthrax outbreak in Sverdlovsk10,20 and observations from the 2001 US outbreak20. Estimates of λ, based on estimates of the ID(50) in experimental studies with rhesus macaque monkeys, suggest that λ ranges between 3.2 × 10-6 and 8.1 × 10-5 per day27,28. Thus, λ is much smaller than θ. The dose to cause disease with probability p = P/100 is called the infectious dose ID(P) and is given by20 D = -(λ + θ)ln(1 - p)/λ. Substituting this expression for D into the formula for c and approximating λ + θ ≈ θ, we find

Thus, we have the surprising mathematical result that it is not necessary to have a precise value for λ to obtain our key results.
We divide the population into subgroups in which the periods of disease protection are the same for all persons in a subgroup. For example, consider a subgroup in which antibiotic protection begins at time t1 and stops at time t2, then we set t3 to infinity in equation (1). Suppose further that post-exposure vaccine protection is achieved at tv, which occurs before antibiotic protection stops (tv < t2), then t2 and t3 are set to infinity. If vaccine protection occurs after antibiotic protection stops then t3 is set equal to tv. The cumulative probability of disease in the population (c) is then a weighted average of the values of c for each subgroup, where the weights are the proportions of people in subgroups. These proportions are determined by the distributions of times in which vaccine immunity is achieved, and the antibiotic start and stop dates (determined by antibiotic adherence rates and efficacy). Among persons exposed to the ID(P), the percentage of cases prevented with post-exposure interventions is:

Impact of pre-exposure vaccination
The impact of adding a pre-exposure vaccination programme is to reduce the cumulative probability of disease from c to c(1 - βφ), in which φ is the vaccine efficacy (that is, the probability that the vaccine protects from disease), and β is the vaccine coverage (that is, the fraction of the population that receives pre-exposure vaccination). The fraction r of all cases prevented by adding a pre-exposure vaccination programme to the post-exposure prophylaxis strategy among persons exposed to the ID(P), is:

If we solve equation (3) for β we obtain β = {1 - [(p(1 - r))/c]}φ-1.
The above equation for β was used to calculate the vaccine coverage rates given in Table 3 with r = 0.75 and 0.90.
Shortening antibiotic regimen by post-exposure vaccine
Consider two situations. In situation 1, antibiotics stop at time tv, at which time vaccine protection begins with probability φ. In situation 2, antibiotics are continued for a longer period of time, until t2, but there is no vaccine protection. We calculate by how much time antibiotics can be shortened (t2 - tv) in order that the disease risks in the two situations are equal. We set the two probabilities of disease conditional on being disease free at tv equal to each other. Solving that equation and approximating λ + θ ≈ θ, we obtain t2 - tv = [- ln(1 - φ)]/θ; this was used to produce Fig. 2.
References
Inglesby, T. V. et al. Anthrax as a biological weapon: medical and public health management. J. Am. Med. Assoc. 281, 1735–1745 (1999)
Inglesby, T. V. et al. Anthrax as a biological weapon. Updated recommendations for management. J. Am. Med. Assoc. 287, 2236–2252 (2002)
Centers for Disease Control. Notice to readers: use of anthrax vaccine in response to terrorism: supplemental recommendations of the Advisory Committee on Immunization Practices. Morb. Mortal. Wkly Rep. 51, 1024–1026 (2002)
Jernigan, J. A. et al. Bioterrorism-related inhalation anthrax: The first 10 cases reported in the United States. Emerg. Infect. Dis. 17, 933–944 (2001)
Joellenbeck, L., Zwanziger, L., Durch, J. & Strom, B. (eds) The Anthrax Vaccine: Is it Safe? Does it Work? (National Academy Press, Washington DC, 2002)
National Institute of Allergy and Infectious Diseases. National Institute of Allergy and Infectious Diseases biodefense research agenda for CDC category A agents. 〈http://www.niaid.nih.gov/publications/bioterrorism.htm〉 (2002).
Centers for Disease Control. Update: investigation of bioterrorism-related anthrax. Morb. Mortal. Wkly Rep. 50, 1008–1010 (2001)
Shepard, C. W. et al. Antimicrobial post-exposure prophylaxis for anthrax: Adverse events and adherence. Emerg. Infect. Dis. 8, 1124–1132 (2002)
Brookmeyer, R. & Blades, N. Prevention of inhalational anthrax in the U.S. outbreak. Science 295, 1861 (2002)
Meselson, M. et al. The Sverdlovsk anthrax outbreak of 1979. Science 266, 1202–1208 (1994)
Wein, L. M., Craft, D. L. & Kaplan, E. H. Emergency response to an anthrax outbreak. Proc. Natl Acad. Sci. USA 100, 4346–4351 (2003)
Committee on Research and Development Needs for Improving Civilian Medical Response to Chemical and Biological Terrorism Incidents. Chemical and Biological Terrorism (National Academy Press, Washington DC, 1999)
Kaplan, E. H., Craft, D. L. & Wein, L. M. Emergency response to a smallpox attack: the case for mass vaccination. Proc. Natl Acad. Sci. USA 99, 10935–10940 (2002)
Bozette, S. et al. A model for a smallpox-vaccination policy. N. Engl. J. Med. 348, 416–425 (2003)
Halloran, M. E., Longini, I. M. Jr, Nizam, A. & Yang, Y. Containing bioterrorist smallpox. Science 298, 1428–1432 (2002)
Ferguson, N. M. et al. Planning for smallpox outbreak. Nature 425, 681–685 (2003)
Fraser, C., Riley, S., Anderson, R. M. & Ferguson, N. M. Factors that make an infectious disease outbreak controllable. Proc. Natl Acad. Sci. USA 101, 6146–6151 (2004)
Kaufmann, A., Meltzer, M. & Schmid, G. The economic impact of a bioterrorist attack: Are prevention and post attack intervention programs justifiable? Emerg. Infect. Dis. 3, 83–94 (1997)
Centers for Disease Control and Prevention. Mass antibiotic dispensing: A primer. 〈http://www.phppo.cdc.gov/phtn/antibiotic/default.asp〉 (June 2004).
Brookmeyer, R., Johnson, E. & Bollinger, R. Modeling the optimum duration of antibiotic prophylaxis in an anthrax outbreak. Proc. Natl Acad. Sci. USA 100, 10129–10132 (2003)
Brachman, P. S. Inhalation anthrax. Ann. NY Acad. Sci. 353, 83–93 (1980)
Ross, J. M. The pathogenesis of anthrax following the administration of spores by the respiratory route. J. Pathol. Bacteriol. 73, 484–494 (1957)
Friedlander, A. M. et al. Post-exposure prophylaxis against experimental inhalation anthrax. J. Infect. Dis. 167, 1239–1242 (1993)
Friedlander, A. M. Anthrax: clinical features, pathogenesis and potential biological warfare threat. Curr. Clin. Top. Infect. Dis. 20, 335–349 (2000)
Cieslak, T. J. & Edward, M. E. Clinical and epidemiological principles of anthrax. Infect. Dis. 5, 51–55 (1999)
Henderson, D. W., Peakcock, S. & Belton, F. C. Observations on the prophylaxis of experimental pulmonary anthrax in the monkey. J. Hyg. (Lond.) 54, 28–35 (1956)
Haas, C. N. On the risk of mortality to primates exposed to anthrax spores. Risk Anal. 22, 189–193 (2002)
Watson, A. & Keir, D. Information on which to base assessments of risk from environmental contaminated with anthrax spores. Epidemiol. Infect. 113, 479–490 (1994)
Acknowledgements
The authors acknowledge the comments of the Anthrax Modeling Working Group of the Secretary's Council on Public Health Preparedness of the Department of Health and Human Services. This research was partially funded by the Fogarty International Center and a grant from the National Institute of Allergy and Infectious Diseases.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Brookmeyer, R., Johnson, E. & Bollinger, R. Public health vaccination policies for containing an anthrax outbreak. Nature 432, 901–904 (2004). https://doi.org/10.1038/nature03087
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03087
This article is cited by
-
?Don't mass vaccinate? against anthrax
Nature (2004)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.