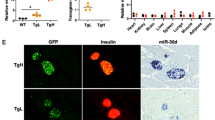
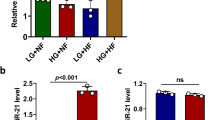
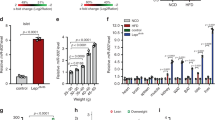
Abstract
MicroRNAs (miRNAs) constitute a growing class of non-coding RNAs that are thought to regulate gene expression by translational repression1. Several miRNAs in animals exhibit tissue-specific or developmental-stage-specific expression, indicating that they could play important roles in many biological processes2,3,4. To study the role of miRNAs in pancreatic endocrine cells we cloned and identified a novel, evolutionarily conserved and islet-specific miRNA (miR-375). Here we show that overexpression of miR-375 suppressed glucose-induced insulin secretion, and conversely, inhibition of endogenous miR-375 function enhanced insulin secretion. The mechanism by which secretion is modified by miR-375 is independent of changes in glucose metabolism or intracellular Ca2+-signalling but correlated with a direct effect on insulin exocytosis. Myotrophin (Mtpn) was predicted to be and validated as a target of miR-375. Inhibition of Mtpn by small interfering (si)RNA mimicked the effects of miR-375 on glucose-stimulated insulin secretion and exocytosis. Thus, miR-375 is a regulator of insulin secretion and may thereby constitute a novel pharmacological target for the treatment of diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bartel, D. P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 (2004)
Lagos-Quintana, M., Rauhut, R., Lendeckel, W. & Tuschl, T. Identification of novel genes coding for small expressed RNAs. Science 294, 853–858 (2001)
Lau, N. C., Lim, L. P., Weinstein, E. G. & Bartel, D. P. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science 294, 858–862 (2001)
Lee, R. C. & Ambros, V. An extensive class of small RNAs in Caenorhabditis elegans. Science 294, 862–864 (2001)
Carrington, J. C. & Ambros, V. Role of microRNAs in plant and animal development. Science 301, 336–338 (2003)
Wightman, B., Ha, I. & Ruvkun, G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75, 855–862 (1993)
Olsen, P. H. & Ambros, V. The lin-4 regulatory RNA controls developmental timing in Caenorhabditis elegans by blocking LIN-14 protein synthesis after the initiation of translation. Dev. Biol. 216, 671–680 (1999)
Brennecke, J., Hipfner, D. R., Stark, A., Russell, R. B. & Cohen, S. M. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell 113, 25–36 (2003)
Xu, P., Vernooy, S. Y., Guo, M. & Hay, B. A. The Drosophila microRNA Mir-14 suppresses cell death and is required for normal fat metabolism. Curr. Biol. 13, 790–795 (2003)
Pfeffer, S., Lagos-Quintana, M. & Tuschl, T. in Current Protocols in Molecular Biology (eds Ausubel, F. M. B. R. et al.) Ch. 26.4.1–26.4.18 (Wiley Interscience, New York, 2003)
Ambros, V. et al. A uniform system for microRNA annotation. RNA 9, 277–279 (2003)
Meister, G., Landthaler, M., Dorsett, Y. & Tuschl, T. Sequence-specific inhibition of microRNA- and siRNA-induced RNA silencing. RNA 10, 544–550 (2004)
Hutvagner, G., Simard, M. J., Mello, C. C. & Zamore, P. D. Sequence-specific inhibition of small RNA function. PLoS Biol 2, E98 (2004)
Barg, S., Galvanovskis, J., Gopel, S. O., Rorsman, P. & Eliasson, L. Tight coupling between electrical activity and exocytosis in mouse glucagon-secreting alpha-cells. Diabetes 49, 1500–1510 (2000)
Gopel, S. O., Kanno, T., Barg, S. & Rorsman, P. Patch-clamp characterisation of omatostatin-secreting cells in intact mouse pancreatic islets. J. Physiol. 528, 497–507 (2000)
Tsuboi, T., da Silva Xavier, G., Leclerc, I. & Rutter, G. A. 5′-AMP-activated protein kinase controls insulin-containing secretory vesicle dynamics. J. Biol. Chem. 278, 52042–52051 (2003)
Rajewsky, N. & Socci, N. D. Computational identification of microRNA targets. Dev. Biol. 267, 529–535 (2004)
Antonin, W., Riedel, D. & von Mollard, G. F. The SNARE Vti1a-β is localized to small synaptic vesicles and participates in a novel SNARE complex. J. Neurosci. 20, 5724–5732 (2000)
Taoka, M. et al. V-1, a protein expressed transiently during murine cerebellar development, regulates actin polymerization via interaction with capping protein. J. Biol. Chem. 278, 5864–5870 (2003)
Larsen, C. M. et al. Interleukin-1β-induced rat pancreatic islet nitric oxide synthesis requires both the p38 and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinases. J. Biol. Chem. 273, 15294–15300 (1998)
Zhao, C., Wilson, M. C., Schuit, F., Halestrap, A. P. & Rutter, G. A. Expression and distribution of lactate/monocarboxylate transporter isoforms in pancreatic islets and the exocrine pancreas. Diabetes 50, 361–366 (2001)
Schreiber-Agus, N. et al. An amino-terminal domain of Mxi1 mediates anti-Myc oncogenic activity and interacts with a homolog of the yeast transcriptional repressor SIN3. Cell 80, 777–786 (1995)
Yamakuni, T. et al. V-1, a catecholamine biosynthesis regulatory protein, positively controls catecholamine secretion in PC12D cells. FEBS Lett. 530, 94–98 (2002)
Lewis, B. P., Shih, I. H., Jones-Rhoades, M. W., Bartel, D. P. & Burge, C. B. Prediction of mammalian microRNA targets. Cell 115, 787–798 (2003)
Lagos-Quintana, M. et al. Identification of tissue-specific microRNAs from mouse. Curr. Biol. 12, 735–739 (2002)
Minami, K. et al. Insulin secretion and differential gene expression in glucose-responsive and -unresponsive MIN6 sublines. Am. J. Physiol. Endocrinol. Metab. 279, E773–E781 (2000)
Shih, D. Q. et al. Profound defects in pancreatic beta-cell function in mice with combined heterozygous mutations in Pdx-1, Hnf-1α, and Hnf-3β. Proc. Natl Acad. Sci. USA 99, 3818–3823 (2002)
Eliasson, L. et al. SUR1 regulates PKA-independent cAMP-induced granule priming in mouse pancreatic B-cells. J. Gen. Physiol. 121, 181–197 (2003)
Olofsson, C. S. et al. Fast insulin secretion reflects exocytosis of docked granules in mouse pancreatic B-cells. Pflugers Arch. 444, 43–51 (2002)
Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31, 3406–3415 (2003)
Acknowledgements
We thank K. Borglid, J. Chen, J. Galvanovskis, M. Lagos-Quintana, M. Landthaler, A. Lingqvist, G. Meister, B. M. Nilsson, A. Wendt and C. Wolfrum for advice and technical assistance. This work was supported by an unrestricted grant from Bristol Myers Squibb, the Juvenile Diabetes Research Foundation, the Deutsche Forschungsgemeinschaft and grants from the Swedish Research Council, the Swedish Diabetes Association, the Göran Gustafsson Stiftelse for Natural Sciences and Medicine and the Swedish Strategic Research Foundation (SSF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Figure 1
Oligoribonucleotide 2’-O-me-375 anneals to endogenous miR-375 in MIN6 cells. Northern blot of MIN6 cells that were transfected with either 2’O-me-eGFP (control) or 2’-O-me-375. (PDF 84 kb)
Supplementary Figure 2
Increased [ATP]i levels in MIN6 cells infected with Ad-375 compared to Ad-eGFP. Total ATP measurements in MIN6 cells that were transfected with Ad-375 or Ad-eGFP. (PDF 42 kb)
Supplementary Figure 3
No apparent effects of miR-375 overexpression on submembrane actin network. Structural analysis using fluorescence microscopy of the submembrane actin network in MIN6 cells and primary β-cells that were infected with Ad-eGFP or Ad-375. (PDF 717 kb)
Supplementary Figure 4
Increased number of docked granules in Ad-375 infected β-cells. Electron micrographs of β-cells infected with Ad-eGFP or Ad-375 and quantitative analysis of granule density. (PDF 855 kb)
Supplementary Table 1
Mature and precursor sequences of mouse (mmu-miR) and human (hsa-miR) novel miRNAs, identified in mouse pancreatic α- and β-cell lines TC1 and MIN6, respectively. (DOC 182 kb)
Supplementary Table 2
Foldback precursor sequences of novel mouse miRNAs, identified in mouse pancreatic β-cell line MIN6. (DOC 24 kb)
Supplementary Table 3
Similar [Ca++]i in primary β-cells and MIN6 cells infected with Ad-GFP and Ad-375. Ca2+-measurements in primary β-cells and MIN6 cells infected with Ad-GFP and Ad-375 in response to 25 mM glucose and to 30 mM K+. (DOC 23 kb)
Supplementary Table 4
Similar [Ca++]i in response to glucose and K+ in primary b-cells and MIN6 cells infected with control siRNAs, si-375 and si-Mtpn. Ca2+-measurements in MIN6 cells co-transfected with CMV-eGFP and si-ApoM (control), si-375 or si-Mtpn in response to glucose K+. (DOC 20 kb)
Supplementary Methods
Description of methods used to identify miR-375 targets. (DOC 24 kb)
Rights and permissions
About this article
Cite this article
Poy, M., Eliasson, L., Krutzfeldt, J. et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature 432, 226–230 (2004). https://doi.org/10.1038/nature03076
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03076
This article is cited by
-
Altered expression of miR-375 and miR-541 in type 2 diabetes patients with and without coronary artery disease (CAD): the potential of miR-375 as a CAD biomarker
Journal of Diabetes & Metabolic Disorders (2024)
-
In diabetic male Wistar rats, quercetin-conjugated superparamagnetic iron oxide nanoparticles have an effect on the SIRT1/p66Shc-mediated pathway related to cognitive impairment
BMC Pharmacology and Toxicology (2023)
-
Clustering pattern and evolution characteristic of microRNAs in grass carp (Ctenopharyngodon idella)
BMC Genomics (2023)
-
An integrated multi-omic approach demonstrates distinct molecular signatures between human obesity with and without metabolic complications: a case–control study
Journal of Translational Medicine (2023)
-
Directed self-assembly of a xenogeneic vascularized endocrine pancreas for type 1 diabetes
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.