Abstract
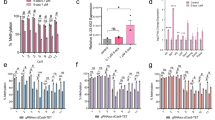
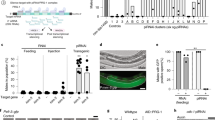
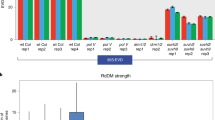
Double-stranded RNAs (dsRNAs) induce post-transcriptional gene silencing in several species of animal and plant1,2. In plants, dsRNAs targeted to CpG islands within a promoter can also induce RNA-directed DNA methylation3,4,5,6,7,8; however, it remains unclear whether gene silencing mediated by DNA methylation can be induced by dsRNAs in mammalian cells. Here, we demonstrate that short interfering RNAs (siRNAs; 21–25-nucleotide RNA molecules) induce DNA methylation and histone H3 methylation in human cells. Synthetic siRNAs targeted to CpG islands of an E-cadherin promoter induced significant DNA methylation and histone H3 lysine 9 methylation in both MCF-7 and normal mammary epithelial cells. As a result, these siRNAs repressed expression of the E-cadherin gene at the transcriptional level. In addition, disrupting the expression of either one of two DNA methyltransferases (DNMT1 or DNMT3B) by specific siRNAs abolished the siRNA-mediated methylation of DNA. Moreover, vector-based siRNAs targeted to the erbB2 (also known as HER2) promoter also induced DNA methylation in MCF-7 cells. Thus, siRNAs targeted to CpG islands within the promoter of a specific gene can induce transcriptional gene silencing by means of DNA-methyltransferase-dependent methylation of DNA in human cells, and might have potential as a new type of gene therapeutic agent.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fire, A. et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806–811 (1998)
Hutvagner, G. & Zamore, P. D. RNAi: nature abhors a double-strand. Curr. Opin. Genet. Dev. 12, 225–232 (2002)
Pelissier, T. & Wassenegger, M. A DNA target of 30 bp is sufficient for RNA-directed DNA methylation. RNA 6, 55–65 (2000)
Mette, M. F., Aufsatz, W., van der Winden, J., Matzke, M. A. & Matzke, A. J. Transcriptional silencing and promoter methylation triggered by double-stranded RNA. EMBO J. 19, 5194–5201 (2000)
Jones, L., Ratcliff, F. & Baulcombe, D. C. RNA-directed transcriptional gene silencing in plants can be inherited independently of the RNA trigger and requires Met1 for maintenance. Curr. Biol. 11, 747–757 (2001)
Hamilton, A., Voinnet, O., Chappell, L. & Baulcombe, D. Two classes of short interfering RNA in RNA silencing. EMBO J. 21, 4671–4769 (2002)
Aufsatz, W., Mette, M. F., Van Der Winden, J., Matzke, M. & Matzke, A. J. HDA6, a putative histone deacetylase needed to enhance DNA methylation induced by double-stranded RNA. EMBO J. 21, 6832–6841 (2002)
Zilberman, D., Cao, X. & Jacobsen, S. E. ARGONAUTE4 control of locus-specific siRNA accumulation and DNA and histone methylation. Science 299, 716–719 (2003)
Bernstein, E., Caudy, A. A., Hammond, S. M. & Hannon, G. J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409, 363–366 (2001)
Elbashir, S. M., Lendeckel, W. & Tuschl, T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 15, 188–200 (2001)
Zamore, P., Tuschl, T., Sharp, P. & Bartel, D. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21- to 23-nucleotide intervals. Cell 101, 25–33 (2000)
Hammond, S. M., Bernstein, E., Beach, D. & Hannon, G. J. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 404, 293–296 (2000)
Elbashir, S. M. et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in mammalian cell culture. Nature 411, 494–498 (2001)
Hutvagner, G. & Zamore, P. D. A microRNA in a multiple-turnover RNAi enzyme complex. Science 297, 2056–2060 (2002)
Zeng, Y. & Cullen, B. R. RNA interference in human cells is restricted to the cytoplasm. RNA 8, 855–860 (2002)
Kawasaki, H. & Taira, K. Short hairpin type of dsRNAs that are controlled by tRNAVal promoter significantly induce RNAi-mediated gene silencing in the cytoplasm of human cells. Nucleic Acids Res. 31, 700–707 (2003)
Herman, J. G., Graff, J. R., Myohanen, S., Nelkin, B. D. & Baylin, S. B. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc. Natl Acad. Sci. USA 93, 9821–9826 (1996)
Graff, J. R., Herman, J. G., Myohanen, S., Baylin, S. B. & Vertino, P. M. Mapping patterns of CpG island methylation in normal and neoplastic cells implicates both upstream and downstream regions in de novo methylation. J. Biol. Chem. 272, 22322–22329 (1997)
Corn, P. G. et al. E-cadherin expression is silenced by 5′ CpG island methylation in acute leukemia. Clin. Cancer Res. 6, 4243–4248 (2000)
Volpe, T. A. et al. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science 297, 1833–1837 (2002)
Pal-Bhadra, M. et al. Heterochromatic silencing and HP1 localization in Drosophila are dependent on the RNAi machinery. Science 303, 669–672 (2004)
Maison, C. et al. Higher-order structure in pericentric heterochromatin involves a distinct pattern of histone modification and an RNA component. Nature Genet. 30, 329–334 (2002)
Bestor, T. H. The DNA methyltransferases of mammals. Hum. Mol. Genet. 9, 2395–2402 (2000)
Rhee, I. et al. DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature 416, 552–556 (2002)
Robert, M. F. et al. DNMT1 is required to maintain CpG methylation and aberrant gene silencing in human cancer cells. Nature Genet. 33, 61–65 (2003)
Hattori, M., Sakamoto, H., Satoh, K. & Yamamoto, T. DNA demethylase is expressed in ovarian cancers and the expression correlates with demethylation of CpG sites in the promoter region of c-erbB-2 and survivin genes. Cancer Lett. 169, 155–164 (2001)
Li, E. Chromatin modification and epigenetic reprogramming in mammalian development. Nature Rev. Genet. 3, 662–673 (2002)
Esteller, M. CpG island hypermethylation and tumor suppressor genes: a booming present, a brighter future. Oncogene 21, 5427–5440 (2002)
Morel, J. B., Mourrain, P., Beclin, C. & Vaucheret, H. DNA methylation and chromatin structure affect transcriptional and post-transcriptional transgene silencing in Arabidopsis. Curr. Biol. 10, 1591–1594 (2000)
Beclin, C., Boutet, S., Waterhouse, P. & Vaucheret, H. A branched pathway for transgene-induced RNA silencing in plants. Curr. Biol. 12, 684–688 (2002)
Acknowledgements
This research was supported by grants from AIST and by a Grant-in-Aid for Scientific Research and for the 21st Century COE programmes, Center for Integrated Brain Medical Science and Human-Friendly Materials based on Chemistry, from the Ministry of Education, Culture, Sports, Science and Culture (MEXT) of Japan.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Figure 1
DNA methylation of the E-cadherin promoter by the mixture of siRNAs (sites 1-10) in MCF-7 cells. (PDF 47 kb)
Supplementary Figure 2
Sites of DNA methylation induced by siRNAs targeted to site 6 and site 10 of the E-cadherin promoter in normal human mammary epithelial cells, as determined by bisulfite sequencing. (PDF 16 kb)
Supplementary Figure 3
Induction of DNA methylation of the erbB2 promoter by the mixture of tRNA-shRNAs (site 1-5). (PDF 37 kb)
Supplementary Methods
This file contains additional Methods (preparation of synthetic siRNAs, construction of tRNA-shRNA expression plasmids, chromatin immunoprecipitaion assay). (RTF 48 kb)
Supplementary Legends
This file contains legends for Supplementary Figs 1–3. (RTF 8 kb)
Rights and permissions
About this article
Cite this article
Kawasaki, H., Taira, K. Induction of DNA methylation and gene silencing by short interfering RNAs in human cells. Nature 431, 211–217 (2004). https://doi.org/10.1038/nature02889
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature02889
This article is cited by
-
Exposure to NO2, CO, and PM2.5 is linked to regional DNA methylation differences in asthma
Clinical Epigenetics (2018)
-
Gene silencing of heparanase results in suppression of invasion and migration of hepatoma cells
World Journal of Surgical Oncology (2014)
-
Development and antitumor activity of a BCL-2 targeted single-stranded DNA oligonucleotide
Cancer Chemotherapy and Pharmacology (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.