Abstract
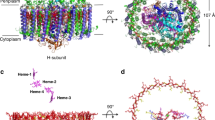
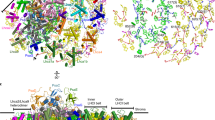
The major light-harvesting complex of photosystem II (LHC-II) serves as the principal solar energy collector in the photosynthesis of green plants and presumably also functions in photoprotection under high-light conditions. Here we report the first X-ray structure of LHC-II in icosahedral proteoliposome assembly at atomic detail. One asymmetric unit of a large R32 unit cell contains ten LHC-II monomers. The 14 chlorophylls (Chl) in each monomer can be unambiguously distinguished as eight Chla and six Chlb molecules. Assignment of the orientation of the transition dipole moment of each chlorophyll has been achieved. All Chlb are located around the interface between adjacent monomers, and together with Chla they are the basis for efficient light harvesting. Four carotenoid-binding sites per monomer have been observed. The xanthophyll-cycle carotenoid at the monomer–monomer interface may be involved in the non-radiative dissipation of excessive energy, one of the photoprotective strategies that have evolved in plants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Peter, G. F. & Thornber, J. P. Biochemical composition and organization of higher plant photosystem II light-harvesting pigment-proteins. J. Biol. Chem. 266, 16745–16754 (1991)
Ruban, A. V., Lee, P. J., Wentworth, M., Young, A. J. & Horton, P. Determination of the stoichiometry and strength of binding of xanthophylls to the photosystem II light harvesting complexes. J. Biol. Chem. 274, 10458–10465 (1999)
Nußberger, S., Dörr, K., Wang, D. N. & Kühlbrandt, W. Lipid–protein interactions in crystals of plant light-harvesting complex. J. Mol. Biol. 234, 347–356 (1993)
Horton, P., Ruban, A. V. & Walters, R. G. Regulation of light harvesting in green plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 655–684 (1996)
Elrad, D., Niyogi, K. K. & Grossman, A. R. A major light-harvesting polypeptide of photosystem II functions in thermal dissipation. Plant Cell 14, 1801–1816 (2002)
Nilsson, A. et al. Phosphorylation controls the three-dimensional structure of plant light harvesting complex II. J. Biol. Chem. 272, 18350–18357 (1997)
Kühlbrandt, W., Wang, D. N. & Fujiyoshi, Y. Atomic model of plant light-harvesting complex by electron crystallography. Nature 367, 614–621 (1994)
Michel, H. Crystallization of membrane proteins. Trends Biochem. Sci. 8, 56–59 (1983)
Rossmann, M. G. in Methods in Macromolecular Crystallography (eds Turk, D. & Johnson, L.) 95–103 (IOS Press, Amsterdam, 2001)
Jordan, P. et al. Three-dimensional structure of cyanobacterial photosystem I at 2.5 Å resolution. Nature 411, 909–917 (2001)
McLuskey, K., Prince, S. M., Cogdell, R. J. & Isaacs, N. W. The crystallographic structure of the B800–820 LH3 light-harvesting complex from the purple bacteria Rhodopseudomonas acidophila strain 7050. Biochemistry 40, 8783–8789 (2001)
Bassi, R., Croce, R., Cugini, D. & Sandonà, D. Mutational analysis of a higher plant antenna protein provides identification of chromophores bound into multiple sites. Proc. Natl Acad. Sci. USA 96, 10056–10061 (1999)
Remelli, R., Varotto, C., Sandonà, D., Croce, R. & Bassi, R. Chlorophyll binding to monomeric light-harvesting complex. A mutation analysis of chromophore-binding residues. J. Biol. Chem. 274, 33510–33521 (1999)
Gradinaru, C. C. et al. The flow of excitation energy in LHCII monomers: implications for the structural model of the major plant antenna. Biophys. J. 75, 3064–3077 (1998)
Bassi, R., Sandonà, D. & Croce, R. Novel aspects of chlorophyll a/b-binding proteins. Physiol. Planta. 100, 769–779 (1997)
Plumley, F. G. & Schmidt, G. W. Reconstitution of chlorophyll a/b light-harvesting complexes: Xanthophyll-dependent assembly and energy transfer. Proc. Natl Acad. Sci. USA 84, 146–150 (1987)
Croce, R., Weiss, S. & Bassi, R. Carotenoid-binding sites of the major light-harvesting complex II of higher plants. J. Biol. Chem. 274, 29613–29623 (1999)
Paulsen, H., Finkenzeller, B. & Kuhlein, N. Pigments induce folding of light-harvesting chlorophyll a/b-binding protein. Eur. J. Biochem. 215, 809–816 (1993)
Hobe, S., Niemeier, H., Bender, A. & Paulsen, H. Carotenoid binding sites in LHCIIb. Relative affinities towards major xanthophylls of higher plants. Eur. J. Biochem. 267, 616–624 (2000)
Croce, R., Remelli, R., Varotto, C., Breton, J. & Bassi, R. The neoxanthin binding site of the major light harvesting complex (LHCII) from higher plants. FEBS Lett. 456, 1–6 (1999)
Gradinaru, C. C., Stokkum, I. H. M. V., Pascal, A. A., Grondelle, R. V. & Amerongen, H. V. Identifying the pathways of energy transfer between carotenoids and chlorophylls in LHCII and CP29. A multicolor, femtosecond pump-probe study. J. Phys. Chem. B 104, 9330–9342 (2000)
Croce, R., Müller, M. G., Bassi, R. & Holzwarth, A. R. Carotenoid-to-chlorophyll energy transfer in recombinant major light-harvesting complex (LHC-II) of higher plants. I. femtosecond transient absorption measurements. Biophys. J. 80, 901–915 (2001)
Hieber, A. D., Bugos, R. C. & Yamamoto, H. Y. Plant lipocalins: violaxanthin de-epoxidase and zeaxanthin epoxidase. Biochim. Biophys. Acta 1482, 84–91 (2000)
Demmig-Adams, B. & Adams, W. W. Photoprotection and other responses of plants to high light stress. Annu. Rev. Plant Physiol. Plant Mol. Biol. 43, 599–626 (1992)
Gilmore, A. M. Mechanistic aspects of xanthophyll cycle-dependent photoprotection in higher plant chloroplasts and leaves. Physiol. Planta. 99, 197–209 (1997)
Frank, H. A. et al. Photophysics of the carotenoids associated with the xanthophyll cycle in photosynthesis. Photosynth. Res. 41, 389–395 (1994)
Ma, Y. Z., Holt, N. E., Li, X. P., Niyogi, K. K. & Fleming, G. R. Evidence for direct carotenoid involvement in the regulation of photosynthetic light harvesting. Proc. Natl Acad. Sci. USA 100, 4377–4382 (2003)
Beddard, G. S., Carlin, S. E. & Porter, G. Concentration quenching of chlorophyll fluorescence in bilayer lipid vesicles and liposomes. Chem. Phys. Lett. 43, 27–32 (1976)
Moya, I., Silvestri, M., Vallon, O., Cinque, G. & Bassi, R. Time-resolved fluorescence analysis of the photosystem II antenna proteins in detergent micelles and liposomes. Biochemistry 40, 12552–12561 (2001)
Lou, S., Wang, K., Zhao, F., Xu, C. & Kuang, T. A comparative study on PS II light harvesting chlorophyll a/b protein complexes between spinach and cucumber. Acta Bot. Sin. 37, 192–197 (1995)
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
Tong, L. & Rossmann, M. G. The locked rotation function. Acta Crystallogr. A 46, 783–792 (1990)
Weeks, C. M. & Miller, R. The design and implementation of SnB v2.0. J. Appl. Crystallogr. 32, 120–124 (1999)
CCP4 Collaborative Computational Project, The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
Jones, T. A. in Molecular Replacement (eds Dodson, E. J., Gover, S. & Wolf, W.) 92–105 (SERC Daresbury Laboratory, Warrington, 1992)
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991)
Mason, J. G. Nucleotide sequence of a cDNA encoding the light-harvesting chlorophyll a/b binding protein from spinach. Nucleic Acids Res. 17, 5387 (1989)
Brünger, A. T. et al. Crystallography and NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998)
Laskowski, R. A., MacArthur, M. W., Moss, D. S. & Thornton, J. M. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26, 283–291 (1993)
Frishman, D. & Argos, P. Knowledge-based secondary structure assignment. Proteins Struct. Funct. Genet. 23, 566–579 (1995)
Kraulis, P. J. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24, 946–950 (1991)
Merritt, E. A. & Murphy, M. E. P. Raster3D version 2.0. A program for photorealistic molecular graphics. Acta Crystallogr. D 50, 869–873 (1994)
Acknowledgements
We thank D. C. Liang and the late P. S. Tang for their efforts in initiating this project; X. C. Gu for discussions; N. Sakabe and K. Sakabe at PF (Tsukuba, Japan) and the staff at BSRF (Beijing, China) for their support during data collection at the synchrotron facilities. This research was financially supported by the National Key Research Development Project of China, the National Natural Science Foundation of China, the National Key Special Research Program and the Knowledge Innovation Program of the Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Tables
Supplementary Table 1: Coordinations of chlorophylls and their interactions with local environments; Supplementary Table 2: Interaction between chlorophylls with strong excitonic coupling; Supplementary Table 3: Interactions between carotenoids and chlorophylls. (DOC 74 kb)
Rights and permissions
About this article
Cite this article
Liu, Z., Yan, H., Wang, K. et al. Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature 428, 287–292 (2004). https://doi.org/10.1038/nature02373
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02373
This article is cited by
-
Ultrafast excited-state dynamics of Luteins in the major light-harvesting complex LHCII
Photochemical & Photobiological Sciences (2024)
-
Long-term light adaptation of light-harvesting and energy-transfer processes in the glaucophyte Cyanophora paradoxa under different light conditions
Photosynthesis Research (2024)
-
Insights into membrane lipids modification in barley leaves as an adaptation mechanism to cold stress
Plant Growth Regulation (2024)
-
Uphill energy transfer mechanism for photosynthesis in an Antarctic alga
Nature Communications (2023)
-
Cryo-EM structures of LHCII in photo-active and photo-protecting states reveal allosteric regulation of light harvesting and excess energy dissipation
Nature Plants (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.