Abstract
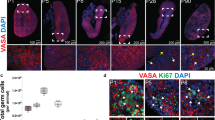
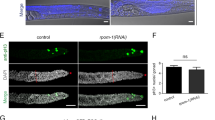
A basic doctrine of reproductive biology is that most mammalian females lose the capacity for germ-cell renewal during fetal life, such that a fixed reserve of germ cells (oocytes) enclosed within follicles is endowed at birth. Here we show that juvenile and adult mouse ovaries possess mitotically active germ cells that, based on rates of oocyte degeneration (atresia) and clearance, are needed to continuously replenish the follicle pool. Consistent with this, treatment of prepubertal female mice with the mitotic germ-cell toxicant busulphan eliminates the primordial follicle reserve by early adulthood without inducing atresia. Furthermore, we demonstrate cells expressing the meiotic entry marker synaptonemal complex protein 3 in juvenile and adult mouse ovaries. Wild-type ovaries grafted into transgenic female mice with ubiquitous expression of green fluorescent protein (GFP) become infiltrated with GFP-positive germ cells that form follicles. Collectively, these data establish the existence of proliferative germ cells that sustain oocyte and follicle production in the postnatal mammalian ovary.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Zuckerman, S. The number of oocytes in the mature ovary. Recent Prog. Horm. Res. 6, 63–108 (1951)
Borum, K. Oogenesis in the mouse. A study of meiotic prophase. Exp. Cell Res. 24, 495–507 (1961)
Peters, H. Migration of gonocytes into the mammalian gonad and their differentiation. Phil. Trans. R. Soc. Lond. B 259, 91–101 (1970)
McLaren, A. Meiosis and differentiation of mouse germ cells. Symp. Soc. Exp. Biol. 38, 7–23 (1984)
Anderson, L. D. & Hirshfield, A. N. An overview of follicular development in the ovary: from embryo to the fertilized ovum in vitro. Md Med. J. 41, 614–620 (1992)
Faddy, M. J., Jones, E. C. & Edwards, R. G. An analytical model for ovarian follicle dynamics. J. Exp. Zool. 197, 173–186 (1976)
Faddy, M. J., Telfer, E. & Gosden, R. G. The kinetics of pre-antral follicle development in ovaries of CBA/Ca mice during the first 14 weeks of life. Cell Tissue Kinet. 20, 551–560 (1987)
Faddy, M. J. Follicle dynamics during ovarian ageing. Mol. Cell. Endocrinol. 163, 43–48 (2000)
Perez, G. I. et al. Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nature Genet. 21, 200–203 (1999)
Tilly, J. L. Commuting the death sentence: how oocytes strive to survive. Nature Rev. Mol. Cell Biol. 2, 838–848 (2001)
Gosden, R. G., Laing, S. C., Felicio, L. S., Nelson, J. F. & Finch, C. E. Imminent oocyte exhaustion and reduced follicular recruitment mark the transition to acyclicity in aging C57BL/6J mice. Biol. Reprod. 28, 255–260 (1983)
Richardson, S. J., Senikas, V. & Nelson, J. F. Follicular depletion during the menopausal transition: evidence for accelerated loss and ultimate exhaustion. J. Clin. Endocrinol. Metab. 65, 1231–1237 (1987)
Lin, H. The tao of stem cells in the germline. Annu. Rev. Genet. 31, 455–491 (1997)
Spradling, A. H., Drummond-Barbosa, D. & Kai, T. Stem cells find their niche. Nature 414, 98–104 (2001)
Lin, H. The stem-cell niche theory: lessons from flies. Nature Rev. Genet. 3, 931–940 (2002)
Wyllie, A. H., Kerr, J. F. R. & Currie, A. R. Cell death: the significance of apoptosis. Int. Rev. Cytol. 68, 251–306 (1980)
Ijiri, K. & Potten, C. S. Response of intestinal cells of differing topographical and hierarchical status to ten cytotoxic drugs and five sources of radiation. Br. J. Cancer 47, 175–185 (1983)
Bursch, W., Paffe, S., Putz, B., Barthel, G. & Schulte-Hermann, R. Determination of the length of the histological stages of apoptosis in normal liver and in altered hepatic foci of rats. Carcinogenesis 11, 847–853 (1990)
Ratts, V. S., Flaws, J. A., Kolp, R., Sorenson, C. M. & Tilly, J. L. Ablation of bcl-2 gene expression decreases the numbers of oocytes and primordial follicles established in the post-natal female mouse gonad. Endocrinology 136, 3665–3668 (1995)
Pepling, M. E. & Spradling, A. C. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev. Biol. 234, 339–351 (2001)
Mattison, D. R. Morphology of oocyte and follicle destruction by polycyclic aromatic hydrocarbons in mice. Toxicol. Appl. Pharmacol. 53, 249–259 (1980)
Matikainen, T. et al. Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nature Genet. 28, 355–360 (2001)
Canning, J., Takai, Y. & Tilly, J. L. Evidence for genetic modifiers of ovarian follicular endowment and development from studies of five inbred mouse strains. Endocrinology 144, 9–12 (2003)
Crone, M., Levy, E. & Peters, H. The duration of the premeiotic DNA synthesis in mouse oocytes. Exp. Cell Res. 39, 678–688 (1965)
Morita, Y. et al. Requirement for phosphatidylinositol-3′-kinase in cytokine-mediated germ cell survival during fetal oogenesis in the mouse. Endocrinology 140, 941–949 (1999)
Fujiwara, Y. et al. Isolation of a DEAD-family protein gene that encodes a murine homolog of Drosophila vasa and its specific expression in germ cell lineage. Proc. Natl Acad. Sci. USA 91, 12258–12262 (1994)
Noce, T., Okamoto-Ito, S. & Tsunekawa, N. Vasa homolog genes in mammalian germ cell development. Cell Struct. Funct. 26, 131–136 (2001)
Selden, J. R. et al. Statistical confirmation that immunofluorescent detection of DNA repair in human fibroblasts by measurement of bromodeoxyuridine incorporation is stoichiometric and sensitive. Cytometry 14, 154–167 (1993)
Davis, A. F. & Clayton, D. A. In situ localization of mitochondrial DNA replication in intact mammalian cells. J. Cell Biol. 135, 883–893 (1996)
Yuan, L. et al. The murine SCP3 gene is required for synaptonemal complex assembly, chromosome synapsis, and male fertility. Mol. Cell 5, 73–83 (2000)
Yuan, L. et al. Female germ cell aneuploidy and embryo death in mice lacking the meiosis-specific protein SCP3. Science 296, 1115–1118 (2002)
Cohen, P. & Pollard, J. W. Regulation of meiotic recombination and prophase I progression in mammals. Bioessays 23, 996–1009 (2001)
Gosden, R., Clarke, H. & Miller, D. in Reproductive Medicine—Molecular, Cellular and Genetic Fundamentals (ed. Fauser, B. C. J. M.) 365–380 (Parthenon, New York, 2003)
Bucci, L. R. & Meistrich, M. L. Effects of busulfan on murine spermatogenesis: cytotoxicity, sterility, sperm abnormalities, and dominant lethal mutations. Mutat. Res. 176, 259–268 (1987)
Brinster, R. L. & Zimmermann, J. W. Spermatogenesis following male germ-cell transplantation. Proc. Natl Acad. Sci. USA 91, 11298–11302 (1994)
Brinster, C. J. et al. Restoration of fertility by germ cell transplantation requires effective recipient preparation. Biol. Reprod. 69, 412–420 (2003)
Pelloux, M. C., Picon, R., Gangnerau, M. N. & Darmoul, D. Effects of busulphan on ovarian folliculogenesis, steroidogenesis and anti-Müllerian activity of rat neonates. Acta Endocrinol. 118, 218–226 (1988)
Generoso, W. M., Stout, S. K. & Huff, S. W. Effects of alkylating agents on reproductive capacity of adult female mice. Mutat. Res. 13, 171–184 (1971)
Shiromizu, K., Thorgeirsson, S. S. & Mattison, D. R. Effect of cyclophosphamide on oocyte and follicle number in Sprague–Dawley rats, C57BL/6N and DBA/2N mice. Pediatr. Pharmacol. 4, 213–221 (1984)
Hadjantonakis, A. K., Gertsenstein, M., Ikawa, M., Okabe, M. & Nagy, A. Generating green fluorescent mice by germline transmission of green fluorescent ES cells. Mech. Dev. 76, 79–90 (1998)
Nagano, M. C. Homing efficiency and proliferation kinetics of male germ line stem cells following transplantation in mice. Biol. Reprod. 69, 701–707 (2003)
Szilvassy, S. J., Ragland, P. L., Miller, C. L. & Eaves, C. J. The marrow homing efficiency of murine hematopoietic stem cells remains constant during ontogeny. Exp. Hematol. 31, 331–338 (2003)
Torrente, Y. et al. Identification of a putative pathway for the muscle homing of stem cells in a muscular dystrophy model. J. Cell Biol. 162, 511–520 (2003)
Oh, H. et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc. Natl Acad. Sci. USA 100, 12313–12318 (2003)
Pearl, R. & Schoppe, W. F. Studies on the physiology of reproduction in the domestic fowl. J. Exp. Zool. 34, 101–118 (1921)
Tilly, J. L. Ovarian follicle counts—not as simple as 1, 2, 3. Reprod. Biol. Endocrinol. 1, 11 (2003)
Walpita, D., Plug, A. W., Neff, N. F., German, J. & Ashley, T. Bloom's syndrome protein, BLM, colocalizes with replication protein A in meiotic prophase nuclei of mammalian spermatocytes. Proc. Natl Acad. Sci. USA 96, 5622–5627 (1999)
Russell, L. B., Hunsicker, P. R., Hack, A. M. & Ashley, T. Effect of the topoisomerase-II inhibitor etoposide on meiotic recombination in male mice. Mutat. Res. 464, 201–212 (2000)
Foley, J. G. D. & Bard, J. B. L. Apoptosis in the cortex of the developing mouse kidney. J. Anat. 201, 477–484 (2002)
Walter, I. et al. Rapid and sensitive detection of enhanced green fluorescent protein expression in paraffin sections by confocal laser scanning microscopy. Histochem. J. 32, 99–103 (2000)
Acknowledgements
We thank Y. Morita for technical assistance; T. Noce for MVH antiserum; T. Ashley for SCP3 antiserum; and I. Schiff and F. Frigoletto Jr for critical reading of the manuscript before its submission. This work was supported by the National Institute on Aging and by Vincent Memorial Research Funds. This study was conducted while J.L.T. was an Investigator of the Steven and Michele Kirsch Foundation, and while J.J. was a Research Fellow supported by The Lalor Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Information
This includes supplementary methods and supplementary figure legends. (DOC 55 kb)
Supplementary Figure 1
Grafted wild-type ovarian tissue adheres to transgenic host ovarian tissue and becomes vascularized. (JPG 48 kb)
Supplementary Figure 2
Wild-type ovarian tissue exhibits a low level of background autofluorescence. (JPG 53 kb)
Supplementary Figure 3
Additional example of folliculogenesis in grafted ovarian tissue. (JPG 31 kb)
Supplementary Figure 4
Postnatal ovarian expression of stem cell-associated genes. (JPG 25 kb)
Rights and permissions
About this article
Cite this article
Johnson, J., Canning, J., Kaneko, T. et al. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 428, 145–150 (2004). https://doi.org/10.1038/nature02316
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02316
This article is cited by
-
Metformin protects ovarian granulosa cells in chemotherapy-induced premature ovarian failure mice through AMPK/PPAR-γ/SIRT1 pathway
Scientific Reports (2024)
-
Exploring the Protective Effect of Total Flavonoids from Semen Cuscutae on Ovarian Germline Stem Cells Based on Notch Signaling Pathway
Stem Cell Reviews and Reports (2024)
-
Chito-oligosaccharides and macrophages have synergistic effects on improving ovarian stem cells function by regulating inflammatory factors
Journal of Ovarian Research (2023)
-
From in vivo to in vitro: exploring the key molecular and cellular aspects of human female gametogenesis
Human Cell (2023)
-
Ovarian aging in humans: potential strategies for extending reproductive lifespan
GeroScience (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.