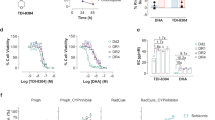
Abstract
Artemisinins are extracted from sweet wormwood (Artemisia annua) and are the most potent antimalarials available1, rapidly killing all asexual stages of Plasmodium falciparum2. Artemisinins are sesquiterpene lactones widely used to treat multidrug-resistant malaria1, a disease that annually claims 1 million lives. Despite extensive clinical and laboratory experience3,4,5 their molecular target is not yet identified. Activated artemisinins form adducts with a variety of biological macromolecules, including haem, translationally controlled tumour protein (TCTP) and other higher-molecular-weight proteins6. Here we show that artemisinins, but not quinine or chloroquine, inhibit the SERCA orthologue (PfATP6) of Plasmodium falciparum in Xenopus oocytes with similar potency to thapsigargin (another sesquiterpene lactone and highly specific SERCA inhibitor). As predicted, thapsigargin also antagonizes the parasiticidal activity of artemisinin. Desoxyartemisinin lacks an endoperoxide bridge and is ineffective both as an inhibitor of PfATP6 and as an antimalarial. Chelation of iron by desferrioxamine abrogates the antiparasitic activity of artemisinins and correspondingly attenuates inhibition of PfATP6. Imaging of parasites with BODIPY-thapsigargin labels the cytosolic compartment and is competed by artemisinin. Fluorescent artemisinin labels parasites similarly and irreversibly in an Fe2+-dependent manner. These data provide compelling evidence that artemisinins act by inhibiting PfATP6 outside the food vacuole after activation by iron.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hien, T. T. & White, N. J. Qinghaosu. Lancet 341, 603–608 (1993)
ter Kuile, F., White, N. J., Holloway, P. H., Pasvol, G. & Krishna, S. Plasmodium falciparum: In vitro studies of the pharmacodynamic properties of drugs used for the treatment of severe malaria. Exp. Parasitol. 76, 85–95 (1993)
Jefford, C. W. Why artemisinin and certain synthetic peroxides are potent antimalarials. Implications for the mode of action. Curr. Med. Chem. 8, 1803–1826 (2001)
Robert, A., Dechy-Cabaret, O., Cazelles, J. & Meunier, B. From mechanistic studies on artemisinin derivatives to new modular antimalarial drugs. Acc. Chem. Res. 35, 167–174 (2002)
Olliaro, P. L., Haynes, R. K., Meunier, B. & Yuthavong, Y. Possible modes of action of the artemisinin-type compounds. Trends Parasitol. 17, 122–126 (2001)
Meshnick, S. R. Artemisinin: mechanisms of action, resistance and toxicity. Int. J. Parasitol. 32, 1655–1660 (2002)
Pandey, A. V., Tekwani, B. L., Singh, R. L. & Chauhan, V. S. Artemisinin, an endoperoxide antimalarial, disrupts the hemoglobin catabolism and heme detoxification systems in malarial parasite. J. Biol. Chem. 274, 19383–19388 (1999)
Haynes, R. K. et al. Artemisinin antimalarials do not inhibit hemozoin formation. Antimicrob. Agents Chemother. 47, 1175 (2003)
O'Neill, P. et al. Biomimetic Fe(II)-mediated degradation of arteflene (Ro-42–1611). The first EPR spin-trapping evidence for the previously postulated secondary carbon-centered cyclohexyl radical. J. Org. Chem. 65, 1578–1582 (2000)
Hawley, S. R. et al. Relationship between antimalarial drug activity, accumulation, and inhibition of heme polymerization in Plasmodium falciparum in vitro. Antimicrob. Agents Chemother. 42, 682–686 (1998)
Ellis, D. S. et al. The chemotherapy of rodent malaria, XXXIX. Ultrastructural changes following treatment with artemisinine of Plasmodium berghei infection in mice, with observations of the localization of [3H]-dihydroartemisinine in P. falciparum in vitro. Ann. Trop. Med. Parasitol. 79, 367–374 (1985)
Krishna, S. et al. Expression and functional characterization of a Plasmodium falciparum Ca2+-ATPase (PfATP4) belonging to a subclass unique to apicomplexan organisms. J. Biol. Chem. 276, 10782–10787 (2001)
Toyoshima, C. & Nomura, H. Structural changes in the calcium pump accompanying the dissociation of calcium. Nature 418, 605–611 (2002)
Price, R. et al. The pfmdr1 gene is associated with a multidrug-resistant phenotype in Plasmodium falciparum from the western border of Thailand. Antimicrob. Agents Chemother. 43, 2943–2949 (1999)
Gu, H. M., Warhurst, D. C. & Peters, W. Uptake of [3H] dihydroartemisinine by erythrocytes infected with Plasmodium falciparum in vitro. Trans. R. Soc. Trop. Med. Hyg. 78, 265–270 (1984)
Haynes, R. K. Artemisinin and derivatives: the future for malaria treatment? Curr. Opin. Infect. Dis. 14, 719–726 (2001)
Meshnick, S. R. et al. Iron-dependent free radical generation from the antimalarial agent artemisinin (qinghaosu). Antimicrob. Agents Chemother. 37, 1108–1114 (1993)
Bray, P. G., Mungthin, M., Ridley, R. G. & Ward, S. A. Access to hematin: the basis of chloroquine resistance. Mol. Pharmacol. 54, 170–179 (1998)
Simpson, P. B. & Russell, J. T. Role of sarcoplasmic/endoplasmic-reticulum Ca2+-ATPases in mediating Ca2+ waves and local Ca2+-release microdomains in cultured glia. Biochem. J. 325, 239–247 (1997)
Akompong, T., VanWye, J., Ghori, N. & Haldar, K. Artemisinin and its derivatives are transported by a vacuolar-network of Plasmodium falciparum and their anti-malarial activities are additive with toxic sphingolipid analogues that block the network. Mol. Biochem. Parasitol. 101, 71–79 (1999)
Asawamahasakda, W., Ittarat, I., Pu, Y. M., Ziffer, H. & Meshnick, S. R. Reaction of antimalarial endoperoxides with specific parasite proteins. Antimicrob. Agents Chemother. 38, 1854–1858 (1994)
Bhisutthibhan, J. et al. The Plasmodium falciparum translationally controlled tumor protein homolog and its reaction with the antimalarial drug artemisinin. J. Biol. Chem. 273, 16192–16198 (1998)
Ono, T. et al. Degenerative changes in morphology of Plasmodium falciparum induced by artemether in vitro. Jpn J. Parasitol. 40, 587–595 (1991)
Allen, D. G., Eisner, D. A. & Wray, S. C. Birthday present for digitalis. Nature 316, 674–675 (1985)
Schatzmann, H.-J. Herzglykoside als Hemmstoffe für den aktiven Kalium- und Natriumtransport durch die Erythrocytenmembran. Helv. Physiol. Acta 11, 346–354 (1953)
Toyoshima, C., Nakasako, M., Nomura, H. & Ogawa, H. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 Å resolution. Nature 405, 647–655 (2000)
Woodrow, C. J., Penny, J. I. & Krishna, S. Intraerythrocytic Plasmodium falciparum expresses a high-affinity facilitative hexose transporter. J. Biol. Chem. 274, 7272–7277 (1999)
East, J. M. Purification of a membrane protein (Ca2+/Mg2+-ATPase) and its reconstitution into lipid vesicles. Methods Mol. Biol. 27, 87–94 (1994)
Bers, D. M., Patton, C. W. & Nuccitelli, R. A practical guide to the preparation of Ca2+ buffers. Methods Cell Biol. 40, 3–29 (1994)
Berenbaum, M. C. A method for testing for synergy with any number of agents. J. Infect. Dis. 137, 122–130 (1978)
Acknowledgements
We thank T. Joët and A.-C. Uhlemann for discussions, P. Wünnenberg for technical assistance, A. Craig for a P. falciparum cDNA library and K. Tanabe for the P. falciparum genomic clone 3L6. We thank the charity Hope (Wessex Medical Trust) for financial support of J.M. East, and T. Bolton for access to confocal microscopy. U. E.-L. was funded by the Deutsche Forschungsgemeinschaft, S.A.W. and P.G.B. are funded by the Wellcome Trust and S.K. holds an MRC (UK) grant. This paper is dedicated to the memory of G. Cowan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
S.K. acted as a paid scientific advisor to the Medicines for Malaria initiative in the development of artemisins as antimalarials; however, that advisory role has no bearing on this work, and those advised have not influenced this work. The other authors have no competing financial interests.
Rights and permissions
About this article
Cite this article
Eckstein-Ludwig, U., Webb, R., van Goethem, I. et al. Artemisinins target the SERCA of Plasmodium falciparum. Nature 424, 957–961 (2003). https://doi.org/10.1038/nature01813
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01813
This article is cited by
-
Malaria therapeutics: are we close enough?
Parasites & Vectors (2023)
-
How has mass drug administration with dihydroartemisinin-piperaquine impacted molecular markers of drug resistance? A systematic review
Malaria Journal (2022)
-
Artemisinin inhibits neutrophil and macrophage chemotaxis, cytokine production and NET release
Scientific Reports (2022)
-
Ancient plant-like terpene biosynthesis in corals
Nature Chemical Biology (2022)
-
PredAPP: Predicting Anti-Parasitic Peptides with Undersampling and Ensemble Approaches
Interdisciplinary Sciences: Computational Life Sciences (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.