Abstract
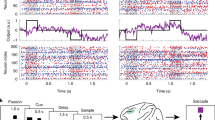
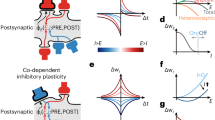
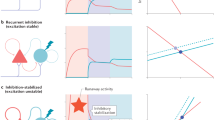
The vast majority of synaptic connections onto neurons in the cerebral cortex arise from other cortical neurons, both excitatory and inhibitory, forming local and distant ‘recurrent’ networks. Although this is a basic theme of cortical organization, its study has been limited largely to theoretical investigations, which predict that local recurrent networks show a proportionality or balance between recurrent excitation and inhibition, allowing the generation of stable periods of activity1,2,3,4,5. This recurrent activity might underlie such diverse operations as short-term memory4,6,7, the modulation of neuronal excitability with attention8,9, and the generation of spontaneous activity during sleep5,10,11,12,13,14. Here we show that local cortical circuits do indeed operate through a proportional balance of excitation and inhibition generated through local recurrent connections, and that the operation of such circuits can generate self-sustaining activity that can be turned on and off by synaptic inputs. These results confirm the long-hypothesized role of recurrent activity as a basic operation of the cerebral cortex.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wang, X. J. Synaptic reverberation underlying mnemonic persistent activity. Trends Neurosci. 24, 455–463 (2001)
van Vreeswijk, C. & Sompolinsky, H. Chaotic balanced state in a model of cortical circuits. Neural Comput. 10, 1321–1371 (1998)
Shadlen, M. N. & Newsome, W. T. The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J. Neurosci. 18, 3870–3896 (1998)
Durstewitz, D., Seamans, J. K. & Sejnowski, T. J. Neurocomputational models of working memory. Nature Neurosci. 3, 1184–1191 (2000)
Compte, A., Sanchez-Vives, M. V., McCormick, D. A. & Wang, X. J. Cellular and network mechanisms of slow oscillatory activity (<1 Hz) in a cortical network model. J. Neurophysiol. 89, 2707–2725 (2003)
Fuster, J. M. Memory in the Cerebral Cortex (MIT press, Cambridge, Massachusetts, 1995)
Brunel, N. & Wang, X. J. Effects of neuromodulation in a cortical network model of object working memory dominated by recurrent inhibition. J. Comput. Neurosci. 11, 63–85 (2001)
Hahnloser, R. H. R., Douglas, R. J. & Hepp, K. Attentional recruitment of inter-areal recurrent networks for selective gain control. Neural Comput. 14, 1669–1689 (2002)
Chance, F. S., Abbott, L. F. & Reyes, A. D. Gain modulation from background synaptic input. Neuron 35, 773–782 (2002)
Steriade, M., Nunez, A. & Amzica, F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J. Neurosci. 13, 3252–3265 (1993)
Contreras, D., Timofeev, I. & Steriade, M. Mechanisms of long lasting hyperpolarizations underlying slow sleep oscillations in cat corticothalamic networks. J. Physiol. (Lond.) 494, 251–264 (1996)
Steriade, M., Timofeev, I. & Grenier, F. Natural waking and sleep states, a view from inside neocortical neurons. J. Neurophysiol. 85, 1969–1985 (2001)
Sanchez-Vives, M. V. & McCormick, D. A. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nature Neurosci. 3, 1027–1034 (2000)
Cowan, R. L. & Wilson, C. J. Spontaneous firing patterns and axonal projections of single corticostriatal neurons in rat medial agranular cortex. J. Neurophysiol. 71, 17–32 (1994)
Egorov, A. V., Hamam, B. N., Fransen, E., Hasselmo, M. E. & Alonso, A. A. Graded persistent activity in entorhinal cortex neurons. Nature 420, 173–178 (2002)
Compte, A. et al. Temporal fluctuations of mnemonic persistent activity in prefrontal neurons of monkeys during a delayed response task. J. Neurophysiol. (submitted)
Funahashi, S., Bruce, C. J. & Goldman-Rakic, P. S. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J. Neurophysiol. 61, 331–349 (1989)
Borg-Graham, L. J., Monier, C. & Frégnac, Y. Visual input evokes transient and strong shunting inhibition in visual cortical neurons. Nature 393, 369–372 (1998)
Hirsch, J. A. & Gilbert, C. D. Synaptic physiology of horizontal connections in cat's visual cortex. J. Neurosci. 11, 1800–1809 (1991)
Anderson, J. S., Carandini, M. & Ferster, D. Orientation tuning of input conductance, excitation, and inhibition in cat primary visual cortex. J. Neurophysiol. 84, 909–926 (2000)
Hirsch, J. A., Alonso, J. M., Reid, R. C. & Martinez, L. M. Synaptic integration in striate cortical simple cells. J. Neurosci. 18, 9517–9528
Carandini, M. & Heeger, D. J. Summation and division by neurons in primary visual cortex. Science 264, 1333–1336 (1994)
Gutkin, B. S., Laing, C. R., Colby, C. L., Show, C. C. & Ermentrout, G. B. Turning on and off with excitation: the role of spike-timing asynchrony and synchrony in sustained neural activity. J. Comput. Neurosci. 11, 121–134 (2001)
Mao, B. Q., Hamzei-Sichani, F., Aronov, D., Froemke, R. C. & Yuste, R. Dynamics of spontaneous activity in neocortical slices. Neuron 32, 883–898 (2001)
Cossart, R., Aronov, D. & Yuste, R. Attractor dynamics of network UP states in the neocortex. Nature 423, 283–288 (2003)
Bernander, Ö., Douglas, R. J., Martin, K. A. C. & Koch, C. Synaptic background activity influences spatiotemporal integration in single pyramidal cells. Proc. Natl Acad. Sci. USA 88, 11569–11573 (1991)
Anderson, J. S., Lampl, I., Gillespie, D. C. & Ferster, D. The contribution of noise to contrast invariance of orientation tuning in cat visual cortex. Science 290, 1968–1972 (2000)
Hahnloser, R. H., Sarpeshkar, R., Mahowald, M. A., Douglas, R. J. & Seung, H. S. Digital selection and analogue amplification coexist in a cortex-inspired silicon circuit. Nature 405, 947–951 (2000)
Stern, P., Edwards, F. A. & Sakmann, B. Fast and slow components of unitary EPSCs on stellate cells elicited by focal stimulation in slices of rat visual cortex. J. Physiol. (Lond.) 449, 247–278 (1992)
Connors, B. W., Malenka, R. C. & Silva, L. R. Two inhibitory postsynaptic potentials, and GABAA and GABAB receptor-mediated responses in neocortex of rat and cat. J. Physiol. (Lond.) 406, 443–468 (1988)
Acknowledgements
We thank R. Yuste for his comments. This work was supported by the National Institutes of Health (D.A.M.), the Human Frontier Science Program (D.A.M.), and by a fellowship from the Howard Hughes Institute (A.H.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Shu, Y., Hasenstaub, A. & McCormick, D. Turning on and off recurrent balanced cortical activity. Nature 423, 288–293 (2003). https://doi.org/10.1038/nature01616
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01616
This article is cited by
-
On the physiological and structural contributors to the overall balance of excitation and inhibition in local cortical networks
Journal of Computational Neuroscience (2024)
-
Mechanisms underlying pathological cortical bursts during metabolic depletion
Nature Communications (2023)
-
Scale free avalanches in excitatory-inhibitory populations of spiking neurons with conductance based synaptic currents
Journal of Computational Neuroscience (2023)
-
Multilevel and multifaceted brain response features in spiking, ERP and ERD: experimental observation and simultaneous generation in a neuronal network model with excitation–inhibition balance
Cognitive Neurodynamics (2023)
-
Proceedings of the First Pediatric Coma and Disorders of Consciousness Symposium by the Curing Coma Campaign, Pediatric Neurocritical Care Research Group, and NINDS: Gearing for Success in Coma Advancements for Children and Neonates
Neurocritical Care (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.