Abstract
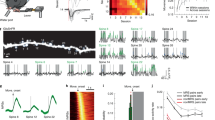
Do new synapses form in the adult cortex to support experience-dependent plasticity? To address this question, we repeatedly imaged individual pyramidal neurons in the mouse barrel cortex over periods of weeks. We found that, although dendritic structure is stable, some spines appear and disappear. Spine lifetimes vary greatly: stable spines, about 50% of the population, persist for at least a month, whereas the remainder are present for a few days or less. Serial-section electron microscopy of imaged dendritic segments revealed retrospectively that spine sprouting and retraction are associated with synapse formation and elimination. Experience-dependent plasticity of cortical receptive fields was accompanied by increased synapse turnover. Our measurements suggest that sensory experience drives the formation and elimination of synapses and that these changes might underlie adaptive remodelling of neural circuits.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Squire, L. R. & Alvarez, P. Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr. Opin. Neurobiol. 5, 169–177 (1995)
Jenkins, W. M., Merzenich, M. M., Ochs, M. T., Allard, T. & Guic-Robles, E. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J. Neurophysiol. 63, 82–104 (1990)
Bakin, J. S. & Weinberger, N. M. Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain. Res. 536, 271–286 (1990)
Gilbert, C. D. & Wiesel, T. N. Receptive field dynamics in adult primary visual cortex. Nature 356, 150–152 (1992)
Diamond, M. E., Huang, W. & Ebner, F. F. Laminar comparison of somatosensory cortical plasticity. Science 265, 1885–1888 (1994)
Florence, S. L., Taub, H. B. & Kaas, J. H. Large-scale sprouting of cortical connections after peripheral injury in adult macaque monkeys. Science 282, 1117–1121 (1998)
Wang, X., Merzenich, M. M., Sameshima, K. & Jenkins, W. M. Remodelling of hand representation in adult cortex determined by timing of tactile stimulation. Nature 378, 71–75 (1995)
Tanzi, E. I fatti i le induzione nell'odierna istologia del sistema nervoso. Riv. Sper. Freniatr. 19, 419–472 (1893)
Martin, S. J., Grimwood, P. D. & Morris, R. G. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu. Rev. Neurosci. 23, 649–711 (2000)
Ramon y Cajal, S. Neue Darstellung vom histologischen Bau des Centralnervensystems. Arch. Anat. Physiol. Anat. Abt. Suppl., 319–428 (1893)
Darian-Smith, C. & Gilbert, C. D. Axonal sprouting accompanies functional reorganization in adult cat striate cortex. Nature 368, 737–740 (1994)
Volkmar, F. R. & Greenough, W. T. Differential rearing effects on rat visual cortical plasticity. Science 176, 1445–1447 (1972)
Ziv, N. E. & Smith, S. J. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron 17, 91–102 (1996)
Stepanyants, A., Hof, P. R. & Chklovskii, D. B. Geometry and structural plasticity of synaptic connectivity. Neuron 34, 275–288 (2002)
Knott, G. W., Quairiaux, C., Genoud, C. & Welker, E. Formation of dendritic spines with GABAergic synapses induced by whisker stimulation in adult mice. Neuron 34, 265–273 (2002)
Bailey, C. H. & Kandel, E. R. Structural changes accompanying memory formation. Annu. Rev. Physiol. 55, 397–426 (1993)
Harris, K. M. Structure, development, and plasticity of dendritic spines. Curr. Opin. Neurobiol. 9, 343–348 (1999)
Nimchinsky, E. A., Sabatini, B. L. & Svoboda, K. Structure and function of dendritic spines. Annu. Rev. Physiol. 64, 313–353 (2002)
Maletic-Savatic, M., Malinow, R. & Svoboda, K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science 283, 1923–1927 (1999)
Engert, F. & Bonhoeffer, T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 399, 66–70 (1999)
Toni, N., Buchs, P. A., Nikonenko, I., Bron, C. R. & Muller, D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature 402, 421–425 (1999)
Yankova, M., Hart, S. A. & Woolley, C. S. Estrogen increases synaptic connectivity between single presynaptic inputs and multiple postsynaptic CA1 pyramidal cells: A serial electron-microscopic study. Proc. Natl Acad. Sci. USA 98, 3525–3530 (2001)
Lendvai, B., Stern, E., Chen, B. & Svoboda, K. Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature 404, 876–881 (2000)
Feng, G. et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 (2000)
Denk, W., Strickler, J. H. & Webb, W. W. Two-photon laser scanning microscopy. Science 248, 73–76 (1990)
Denk, W. & Svoboda, K. Photon upmanship: why multiphoton imaging is more than a gimmick. Neuron 18, 351–357 (1997)
Kim, H. G. & Connors, B. W. Apical dendrites of the neocortex: correlation between sodium- and calcium-dependent spiking and pyramidal cell morphology. J. Neurosci. 13, 5301–5311 (1993)
Vaughn, J. E. & Peters, A. A three dimensional study of layer I of the rat parietal cortex. J. Comp. Neurol. 149, 355–370 (1973)
Svoboda, K., Tank, D. W. & Denk, W. Direct measurement of coupling between dendritic spines and shafts. Science 272, 716–719 (1996)
Harris, K. M., Jensen, F. E. & Tsao, B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J. Neurosci. 12, 2685–2705 (1992)
Wiesel, T. N. The postnatal development of the visual cortex and the influence of environment. Nature 299, 583–591 (1982)
Glazewski, S., McKenna, M., Jacquin, M. & Fox, K. Experience-dependent depression of vibrissae responses in adolescent rat barrel cortex. Eur. J. Neurosci. 10, 2107–2116 (1998)
Fox, K. Anatomical pathways and molecular mechanisms for plasticity in the barrel cortex. Neuroscience 111, 799–814 (2002)
Stern, E., Maravall, M. & Svoboda, K. Rapid development and plasticity of layer 2/3 maps in rat barrel cortex in vivo. Neuron 31, 305–315 (2001)
Zhu, J. J. & Connors, B. W. Intrinsic firing patterns and whisker-evoked synaptic responses of neurons in the rat barrel cortex. J. Neurophysiol. 81, 1171–1183 (1999)
Moore, C. I. & Nelson, S. B. Spatio-temporal subthreshold receptive fields in the vibrissa representation of rat primary somatosensory cortex. J. Neurophysiol. 80, 2882–2892 (1998)
Huttenlocher, P. R., de Courten, C., Garey, L. J. & Van der Loos, H. Synaptogenesis in human visual cortex—evidence for synapse elimination during normal development. Neurosci. Lett. 33, 247–252 (1982)
Rakic, P., Bourgeois, J. P., Eckenhoff, M. F., Zecevic, N. & Goldman-Rakic, P. S. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science 232, 232–235 (1986)
Hubel, D. H. & Wiesel, T. N. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J. Neurophyiol. 26, 1003–1017 (1963)
Antonini, A. & Stryker, M. P. Rapid remodeling of axonal arbors in the visual cortex. Science 260, 1819–1821 (1993)
Sandison, D. R., Piston, D. W. & Webb, W. W. Three-Dimensional Confocal Microscopy: Volume Investigation of Biological Specimens (eds Stevens, J. K., Mills, L. R. & Trogadis, J. E.) 29–47 (Academic, New York, 1994)
Harris, K. M. & Stevens, J. K. Dendritic spines of CA1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characterisitcs. J. Neurosci. 9, 2982–2997 (1989)
Dunaevsky, A., Blazeski, R., Yuste, R. & Mason, C. Spine motility with synaptic contact. Nature Neurosci. 4, 685–686 (2001)
Acknowledgements
We thank M. Chklovskii for useful discussions, E. Ruthazer, W. Thompson, R. Weinberg and members of our laboratory for a critical reading of the manuscript, T. Pologruto and B. Sabatini for programming, and B. Burbach, P. O'Brien and A. Holtmaat for help with experiments. This work was supported by the Pew, Mathers, and Lehrman Foundations, HFSP and NIH (K.S.), and the Swiss National Science Foundation and HFSP (E.W.). B.C. is a predoctoral student at SUNY Stony Brook.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Trachtenberg, J., Chen, B., Knott, G. et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature 420, 788–794 (2002). https://doi.org/10.1038/nature01273
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01273
This article is cited by
-
Real-time visualization of structural dynamics of synapses in live cells in vivo
Nature Methods (2024)
-
Neuro-nanotechnology: diagnostic and therapeutic nano-based strategies in applied neuroscience
BioMedical Engineering OnLine (2023)
-
The times they are a-changin’: a proposal on how brain flexibility goes beyond the obvious to include the concepts of “upward” and “downward” to neuroplasticity
Molecular Psychiatry (2023)
-
Linking spontaneous and stimulated spine dynamics
Communications Biology (2023)
-
Morphological changes of large layer V pyramidal neurons in cortical motor-related areas after spinal cord injury in macaque monkeys
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.