Abstract
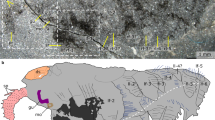
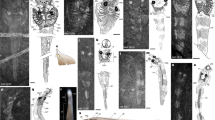
Agnathan fish hold a key position in vertebrate evolution, especially regarding the origin of the head and neural-crest-derived tissue1. In contrast to amphioxus2, lampreys and other vertebrates possess a complex brain and placodes that contribute to well-developed eyes, as well as auditory and olfactory systems3. These sensory sytems were arguably a trigger to subsequent vertebrate diversifications. However, although they are known from skeletal impressions in younger Palaeozoic agnathans4, information about the earliest records of these systems has been largely wanting. Here we report numerous specimens of the Lower Cambrian vertebrate Haikouichthys ercaicunensis, until now only known from the holotype5. Haikouichthys shows significant differences from other fossil agnathans: key features include a small lobate extension to the head, with eyes and possible nasal sacs, as well as what may be otic capsules. A notochord with separate vertebral elements is also identifiable. Phylogenetic analysis indicates that this fish lies within the stem-group craniates. Although Haikouichthys somewhat resembles the ammocoete larva of modern lampreys, this is because of shared general craniate characters; adult lampreys and hagfishes (the cyclostomes if monophyletic6,7) are probably derived in many respects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Northcutt, R. G. The origin of craniates: neural crest, neurogenic placodes, and homeobox genes. Isr. J. Zool. 42, S273–S313 (1996)
Holland, L. Z. & Holland, N. D. Evolution of neural crest and placodes: amphioxus as a model for the ancestral vertebrate? J. Anat. 199, 85–98 (2001)
Kleerekoper, H. The Biology of Lampreys Vol. 2 (eds Hardisty, M. W. & Potter, I. C.) 373–404 (Academic, London, 1972)
Janvier, P. Early Vertebrates (Clarendon, Oxford, 1996)
Shu, D.-G. et al. Lower Cambrian vertebrates from south China. Nature 402, 42–46 (1999)
Mallatt, J. & Sullivan, J. 28S and 18S rDNA sequences support the monophyly of lampreys and hagfishes. Mol. Biol. Evol. 15, 1706–1718 (1998)
Kuraku, S. et al. Monophyly of lampreys and hagfishes supported by nuclear DNA-coded genes. J. Mol. Evol. 49, 729–735 (1999)
Zhang, X.-l. et al. New sites of Chengjiang fossils: crucial windows on the Cambrian explosion. J. Geol. Soc. Lond. 158, 211–218 (2001)
Janvier, P. Catching the first fish. Nature 402, 21–22 (1999)
Shimeld, S. M. & Holland, P. W. H. Vertebrate innovations. Proc. Natl Acad. Sci. USA 97, 4449–4452 (2000)
Holland, H. D. & Chen, J.-y. Origin and early evolution of the vertebrates: new insights from advances in molecular biology, anatomy, and palaeontology. BioEssays 23, 142–151 (2001)
Cole, W. C. & Youson, J. H. Morphology of the pineal complex of the anadromous sea lamprey, Petromyzon marinus L. Am. J. Anat. 165, 131–163 (1982)
Bardack, D. & Zangerl, R. The Biology of Lampreys Vol. 1 (eds Hardisty, M. W. & Potter, I. C.) 67–84 (Academic, London, 1971)
Gabbott, S. E., Aldridge, R. J. & Theron, J. N. A giant conodont with preserved muscle tissue from the Upper Ordovician of South Africa. Nature 374, 800–803 (1995)
Gagnier, P.-Y. Sacabambaspis janvieri, vertébré Ordovicien de Bolivie: I: Analyse morphologique. Ann. Paléontol. 79, 19–51 (1993)
Gagnier, P.-Y. Sacabambaspis janvieri, vertébré Ordovicien de Bolivie 2: Analyse phylogénétique. Ann. Paléontol. 79, 119–166 (1993)
Ritchie, A. New evidence on Jamoytius kerwoodi White, an important ostracoderm from the Silurian of Lanarkshire, Scotland. Palaeontology 11, 21–39 (1968)
Lacalli, T. C. Frontal eye circuitry, rostral sensory pathways and brain organization in amphioxus larvae: evidence from 3D reconstructions. Phil. Trans. R. Soc. Lond. B 351, 243–263 (1996)
Lacalli, T. C. New perspectives on the evolution of protochordate sensory and locomotory systems, and the origin of brains and heads. Phil. Trans. R. Soc. Lond. B 356, 1565–1572 (2001)
Fritzsch, B. Evolution of the vestibulo-ocular system. Otolaryngology Head Neck Surg. 119, 182–192 (1998)
Kuratani, S. et al. Embryology of the lamprey and evolution of the vertebrate jaw: insights from molecular and developmental perspectives. Phil. Trans. R. Soc. Lond. B 356, 1615–1632 (2001)
Cohn, M. J. Lamprey Hox genes and the origin of jaws. Nature 416, 386–387 (2002)
Shigetani, Y. et al. Heterotopic shifts of epithelial–mesenchymal interactions in vertebrate jaw evolution. Science 296, 1316–1319 (2002)
Shu, D.-G. et al. Primitive deuterostomes from the Chengjiang Lagerstätte (Lower Cambrian, China). Nature 414, 419–424 (2001)
Shu, D.-G. et al. An early Cambrian tunicate from China. Nature 411, 472–473 (2001)
Shu, D.-G., Conway Morris, S. & Zhang, X.-L. A Pikaia-like chordate from the Lower Cambrian of China. Nature 384, 157–158 (1996)
Janvier, P. The dawn of the vertebrates: characters versus common ascent in the rise of current vertebrate phylogenies. Palaeontology 39, 259–287 (1996)
Farris, J. S. HENNIG86, version 1.5. Program and user's manual (published by the author, Port Jefferson Station, New York, 1988).
Courrol Ramos, T. TREE GARDENER, version 1.0. 〈http://www.icn.unal.edu.co/extensio/servicio/servicio.html〉 (1996).
Acknowledgements
This work was supported by the Ministry of Sciences and Technology of China, the Natural Science Foundation of China, the Ministry of Education of China, the National Geographic Society (USA), The Royal Society, and St John's College, Cambridge. We thank K. Kardong and B. J. Swalla. L. Guo, X. Cheng, M. Cheng and S. Last are thanked for technical assistance, and Y. Ji and H. Guo for fieldwork help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Shu, DG., Morris, S., Han, J. et al. Head and backbone of the Early Cambrian vertebrate Haikouichthys. Nature 421, 526–529 (2003). https://doi.org/10.1038/nature01264
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01264
This article is cited by
-
Ancestral photoreceptor diversity as the basis of visual behaviour
Nature Ecology & Evolution (2024)
-
Getting inside the oldest known vertebrate skull
Nature (2023)
-
The oldest three-dimensionally preserved vertebrate neurocranium
Nature (2023)
-
Thyroid and endostyle development in cyclostomes provides new insights into the evolutionary history of vertebrates
BMC Biology (2022)
-
Developmental independence of median fins from the larval fin fold revises their evolutionary origin
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.