Abstract
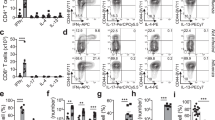
Type-2 immunity requires orchestration of innate and adaptive immune responses to protect mucosal sites from pathogens. Dysregulated type-2 responses result in allergy or asthma1. T helper 2 (TH2) cells elaborate cytokines, such as interleukin (IL)-4, IL-5, IL-9 and IL-13, which work with toxic mediators of innate immune cells to establish environments that are inhospitable to helminth or arthropod invaders2. The importance of TH2 cells in coordinating innate immune cells at sites of inflammation is not known. Here we show that polarized type-2 immune responses are initiated independently of adaptive immunity. In the absence of B and T cells, IL-4-expressing eosinophils were recruited to tissues of mice infected with the helminth Nippostrongylus brasiliensis, but eosinophils failed to degranulate. Reconstitution with CD4 T cells promoted accumulation of degranulated IL-4-expressing cells, but only if T cells were stimulated with cognate antigen. Degranulation correlated with tissue destruction, which was attenuated if eosinophils were depleted. Helper T cells confer antigen specificity on eosinophil cytotoxicity, but not cytokine responses, so defining a novel mechanism that focuses tissue injury at sites of immune challenge.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wills-Karp, M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu. Rev. Immunol. 17, 255–281 (1999)
Finkelman, F. D. et al. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu. Rev. Immunol. 15, 505–533 (1997)
Akira, S. Toll-like receptors and innate immunity. Adv. Immunol. 78, 1–56 (2001)
Schnare, M. et al. Toll-like receptors control activation of adaptive immune responses. Nature. Immunol. 29, 47–50 (2001)
Chin, A. I. et al. Involvement of receptor-interacting protein 2 in innate and adaptive immune responses. Nature 416, 190–194 (2002)
Kobayashi, K. et al. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature 416, 194–199 (2002)
Suzuki, N. et al. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature 416, 750–754 (2002)
Mohrs, M., Shinkai, K., Mohrs, K. & Locksley, R. M. Analysis of type 2 immunity in vivo with a biscistronic reporter. Immunity 15, 303–311 (2001)
Dvorak, A. & Weller, P. F. Ultrastructural analysis of human eosinophils. Chem. Immunol. 76, 1–28 (2000)
McKenzie, G. J., Fallon, P. G., Emson, C. L., Grencis, R. K. & McKenzie, A. N. J. Simultaneous disruption of interleukin (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses. J. Exp. Med. 189, 1565–1572 (1999)
Urban, J. F. Jr et al. IL-13, IL-4Rα, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity 8, 255–264 (1998)
Walsh, G. M. Eosinophil granule proteins and their role in disease. Curr. Opin. Hematol. 8, 28–33 (2001)
Rumbley, C. A. et al. Activated eosinophils are the major source of Th2-associated cytokines in the schistosome granuloma. J. Immunol. 162, 1003–1009 (1999)
Justice, J. P. et al. Ragweed-induced expression of GATA-3, IL-4, and IL-5 by eosinophils in the lungs of allergic C57BL/6J mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 282, L302–L309 (2002)
Foster, P. S. et al. Elemental signals regulating eosinophil accumulation in the lung. Immunol. Rev. 179, 173–181 (2001)
Mould, A. W. et al. The effect of IL-5 and eotaxin expression in the lung on eosinophil trafficking and degranulation and the induction of bronchial hyperreactivity. J. Immunol. 164, 2142–2150 (2000)
Denzler, K. L. et al. Extensive eosinophil degranulation and peroxidase-mediated oxidation of airway proteins do not occur in a mouse ovalbumin-challenge model of pulmonary inflamation. J. Immunol. 167, 1672–1682 (2001)
Erjefalt, J. S. & Persson, C. G. A. New aspects of degranulation and fates of airway mucosal eosinophils. Am. J. Respir. Crit. Care Med. 161, 2074–2085 (2000)
Karawajczyk, M. et al. Piecemeal degranulation of peripheral blood eosinophils. A study of allergic subjects during and out of pollen season. Am. J. Respir. Cell Mol. Biol. 23, 521–529 (2000)
Sabin, E. A., Kopf, M. A. & Pearce, E. J. Schistosoma mansoni egg-induced early IL-4 production is dependent upon IL-5 and eosinophils. J. Exp. Med. 184, 1871–1878 (1996)
Dent, L. A., Strath, M., Mellor, A. L. & Sanderson, C. J. Eosinophilia in transgenic mice expressing interleukin 5. J. Exp. Med. 172, 1425–1431 (1990)
Lee, N. A. et al. Expression of IL-5 in thymocytes/T cells leads to the development of a massive eosinophilia, extramedullary eosinophilopoiesis, and unique histopathologies. J. Immunol. 158, 1332–1344 (1997)
Conrad, D. H., Ben-Sasson, S. Z., Le Gros, G., Finkelman, F. D. & Paul, W. E. Infection with Nippostrongylus brasiliensis or injection with anti-IgD antibodies markedly enhances Fc-receptor-mediated interleukin 4 production by non-B, non-T cells. J. Exp. Med. 171, 1497–1508 (1990)
Dvorak, A. M. Cell biology of the basophil. Int. Rev. Cytol. 180, 87–236 (1998)
Leckie, M. J. et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet 356, 2144–2148 (2000)
Foster, P. S. et al. Interleukin-5 and eosinophils as therapeutic targets for asthma. Trends Mol. Med. 8, 162–167 (2002)
Milgrom, H. et al. Treatment of allergic asthma with monoclonal anti-IgE antibody. rhuMAb-E25 study group. N. Engl. J. Med. 341, 1966–1973 (1999)
Murphy, K. M., Heimberger, A. B. & Loh, D. Y. Induction by antigen of interthymic apoptosis of CD4 + CD8 + TCRlo thymocytes in vivo. Science 250, 1720–1722 (1990)
Fowell, D. J., Magram, J., Turck, C. W., Killeen, N. & Locksley, R. M. Impaired Th2 subset development in the absence of CD4. Immunity 6, 559–569 (1997)
Brown, D. R. et al. Beta-2-microglobulin-dependent NK1.1 + T cells are not essential for T helper cell 2 immune responses. J. Exp. Med. 184, 1295–1304 (1996)
Acknowledgements
The authors thank M.-L. Wong for assistance with electron microscopy, C. McArthur for expertise with cell sorting, San Francisco General Hospital Histopathology Laboratory Service for tissue histology, N. Flores, L. Stowring and Z.-E. Wang for technical assistance, B. Seymour and R. Coffman for parasites, R. Venkayya, D. Erle and A. McKenzie for mice, and J. Bluestone, J. Cyster, M. Kuhns, L. Lanier and laboratory members for critically reading the manuscript. This work was supported in part by funding from National Institute of Allergy and Infectious Diseases and National Heart, Lung and Blood Institute, National Institutes of Health. K.S. is supported by the UCSF Medical Scientist Training Program. R.M.L. is an Ellison Senior Scholar in Global Infectious Diseases.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Shinkai, K., Mohrs, M. & Locksley, R. Helper T cells regulate type-2 innate immunity in vivo. Nature 420, 825–829 (2002). https://doi.org/10.1038/nature01202
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01202
This article is cited by
-
Evidence underscoring immunological and clinical pathological changes associated with Sarcoptes scabiei infection: synthesis and meta-analysis
BMC Infectious Diseases (2022)
-
IL-4 induces reparative phenotype of RPE cells and protects against retinal neurodegeneration via Nrf2 activation
Cell Death & Disease (2022)
-
Repositioning TH cell polarization from single cytokines to complex help
Nature Immunology (2021)
-
Eosinophils and helminth infection: protective or pathogenic?
Seminars in Immunopathology (2021)
-
IL-9 and Th9 in parasite immunity
Seminars in Immunopathology (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.