Abstract
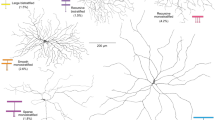
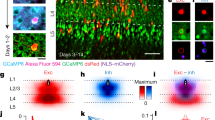
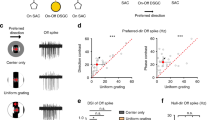
In the retina, directionally selective ganglion cells respond with robust spiking to movement in their preferred direction, but show minimal response to movement in the opposite, or null, direction1,2. The mechanisms and circuitry underlying this computation have remained controversial3. Here we show, by isolating the excitatory and inhibitory inputs to directionally selective cells and measuring direct connections between these cells and presynaptic neurons, that a presynaptic interneuron, the starburst amacrine cell, delivers direct inhibition to directionally selective cells. The processes of starburst cells are connected asymmetrically to directionally selective cells: those pointing in the null direction deliver inhibition; those pointing in the preferred direction do not. Starburst cells project inhibition laterally ahead of a stimulus moving in the null direction. In addition, starburst inhibition is itself directionally selective: it is stronger for movement in the null direction. Excitation in response to null direction movement is reduced by an inhibitory signal acting at a site that is presynaptic to the directionally selective cell. The interplay of these components generates reduced excitation and enhanced inhibition in the null direction, thereby ensuring robust directional selectivity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Barlow, H. B. & Hill, R. M. Selective sensitivity to direction of movement in ganglion cells of rabbit retina. Science 139, 412–414 (1963)
Barlow, H. B. & Levick, W. R. Mechanism of directionally selective units in rabbits retina. J. Physiol. (Lond.) 178, 477–504 (1965)
Vaney, D. I., He, S., Taylor, W. R. & Levick, W. R. Motion Vision—Computational, Neural, and Ecological Constraints (ed. Zeil, J.) 13–56 (Springer, Berlin, 2001)
Kittila, C. A. & Massey, S. C. Pharmacology of directionally selective ganglion cells in the rabbit retina. J. Neurophysiol. 77, 675–689 (1997)
Caldwell, J. H., Daw, N. W. & Wyatt, H. J. Effects of picrotoxin and strychnine on rabbit retinal ganglion-cells—lateral interactions for cells with more complex receptive fields. J. Physiol. (Lond.) 276, 277–298 (1978)
Famiglietti, E. V. Dendritic Costratification of on and on-off directionally selective ganglion-cells with starburst amacrine cells in rabbit retina. J. Comp. Neurol. 324, 322–335 (1992)
Vaney, D. I. & Pow, D. V. The dendritic architecture of the cholinergic plexus in the rabbit retina: selective labeling by glycine accumulation in the presence of sarcosine. J. Comp. Neurol. 421, 1–13 (2000)
O'Malley, D. M., Sandell, J. H. & Masland, R. H. Corelease of acetylcholine and GABA by the starburst amacrine cells. J. Neurosci. 12, 1394–1408 (1992)
Brecha, N., Johnson, D., Peichl, L. & Wässle, H. Cholinergic amacrine cells of the rabbit retina contain glutamate-decarboxylase and γ-aminobutyrate immunoreactivity. Proc. Natl Acad. Sci. USA 85, 6187–6191 (1988)
Vaney, D. I. & Young, H. M. GABA-like immunoreactivity in cholinergic amacrine cells of the rabbit retina. Brain Res. 438, 369–373 (1988)
He, S. G. & Masland, R. H. Retinal direction selectivity after targeted laser ablation of starburst amacrine cells. Nature 389, 378–382 (1997)
Yoshida, K. et al. A key role of starburst amacrine cells in originating retinal directional selectivity and optokinetic eye movement. Neuron 30, 771–780 (2001)
Taylor, W. R., He, S. Y., Levick, W. R. & Vaney, D. I. Dendritic computation of direction selectivity by retinal ganglion cells. Science 289, 2347–2350 (2000)
Borg-Graham, L. J. The computation of directional selectivity in the retina occurs presynaptic to the ganglion cell. Nature Neurosci. 4, 176–183 (2001)
Famiglietti, E. V. Starburst amacrine cells and cholinergic neurons—mirror-symmetric on and off amacrine cells of rabbit retina. Brain Res. 261, 138–144 (1983)
Masland, R. H., Mills, J. W. & Hayden, S. A. Acetylcholine-synthesizing amacrine cells—identification and selective staining by using autoradiography and fluorescent markers. Proc. R. Soc. Lond. Ser. B 223, 79–100 (1984)
Masland, R. H. & Mills, J. W. Autoradiographic identification of acetylcholine in the rabbit retina. J. Cell Biol. 83, 159–178 (1979)
Linn, D. M., Blazynski, C., Redburn, D. A. & Massey, S. C. Acetylcholine-release from the rabbit retina mediated by kainate receptors. J. Neurosci. 11, 111–122 (1991)
Masland, R. H. & Ames, A. Responses to acetylcholine of ganglion-cells in an isolated mammalian retina. J. Neurophysiol. 39, 1220–1235 (1976)
Famiglietti, E. V. Starburst amacrine cells—morphological constancy and systematic variation in the anisotropic field of rabbit retinal neurons. J. Neurosci. 5, 562–577 (1985)
Peters, B. N. & Masland, R. H. Responses to light of starburst amacrine cells. J. Neurophysiol. 75, 469–480 (1996)
Borg-Graham, L. J. & Grzywacz, N. M. Single Neuron Computation (ed. Zornetzer, S. F.) 347–375 (Academic, London, 1992)
Poznanski, R. R. Modeling the electrotonic structure of starburst amacrine cells in the rabbit retina—a functional interpretation of dendritic morphology. Bull. Math. Biol. 54, 905–928 (1992)
Vaney, D. I. Progress in Retinal Research (ed. Chader, G. J.) 49–100 (Oxford, Pergamon, 1990)
Famiglietti, E. V. Synaptic organization of starburst amacrine cells in rabbit retina—analysis of serial thin-sections by electron-microscopy and graphic reconstruction. J. Comp. Neurol. 309, 40–70 (1991)
Euler, T., Detwiler, P. B. & Denk, W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature 418, 845–852 (2002)
Mills, S. L. & Massey, S. C. Morphology of bipolar cells labelled by DAPI in the rabbit retina. J. Comp. Neurol. 321, 133–149 (1992)
Roska, B. & Werblin, F. Vertical interactions across ten parallel, stacked representations in the mammalian retina. Nature 410, 583–587 (2001)
Acknowledgements
We thank K. Okazaki for help with confocal preparations and image analysis; J. Hurtado, R. Kramer and C. Kretschmann for discussion; and H. Barlow, R. Froemke, E. Isacoff, X. Ren and R. Zucker for comments on the manuscript. This work was supported by grants from the Office of Naval Research, the National Eye Institute, and an NIH training grant in Vision Science to the University of California Berkeley (S.F.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Fried, S., Münch, T. & Werblin, F. Mechanisms and circuitry underlying directional selectivity in the retina. Nature 420, 411–414 (2002). https://doi.org/10.1038/nature01179
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01179
This article is cited by
-
Dendritic mGluR2 and perisomatic Kv3 signaling regulate dendritic computation of mouse starburst amacrine cells
Nature Communications (2024)
-
Effective protection of photoreceptors using an inflammation-responsive hydrogel to attenuate outer retinal degeneration
npj Regenerative Medicine (2023)
-
An ON-type direction-selective ganglion cell in primate retina
Nature (2023)
-
Classical center-surround receptive fields facilitate novel object detection in retinal bipolar cells
Nature Communications (2022)
-
Suppression without inhibition: how retinal computation contributes to saccadic suppression
Communications Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.