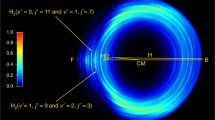
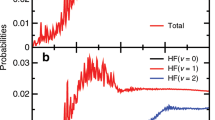
Abstract
Quantum dynamical processes near the energy barrier that separates reactants from products influence the detailed mechanism by which elementary chemical reactions occur. In fact, these processes can change the product scattering behaviour from that expected from simple collision considerations, as seen in the two classical reactions F + H2 → HF + H and H + H2 → H2 + H and their isotopic variants. In the case of the F + HD reaction, the role of a quantized trapped Feshbach resonance state had been directly determined1, confirming previous conclusions2 that Feshbach resonances cause state-specific forward scattering of product molecules. Forward scattering has also been observed in the H + D2 → HD + D reaction3,4 and attributed to a time-delayed mechanism3,5,6,7. But despite extensive experimental8,9,10,11,12 and theoretical13,14,15,16,17,18 investigations, the details of the mechanism remain unclear. Here we present crossed-beam scattering experiments and quantum calculations on the H + HD → H2 + D reaction. We find that the motion of the system along the reaction coordinate slows down as it approaches the top of the reaction barrier, thereby allowing vibrations perpendicular to the reaction coordinate and forward scattering. The reaction thus proceeds, as previously suggested7, through a well-defined ‘quantized bottleneck state’ different from the trapped Feshbach resonance states observed before.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Skodje, R. T. et al. Resonance-mediated chemical reaction: F + HD → HF + D. Phys. Rev. Lett. 85, 1206–1209 (2000)
Neumark, D. M., Wodtke, A. M., Robinson, G. N., Hayden, C. C. & Lee, Y. T. Experimental investigation of resonances in reactive scattering: The F + H2 reaction. Phys. Rev. Lett. 53, 226–229 (1984)
Fernandes-Alonso, F. et al. Evidence for scattering resonances in the H + D2 reaction. Angew. Chem. Int. Edn Engl. 39, 2748–2752 (2001)
Fernandez-Alonso, F. et al. Forward scattering in the H + D2 → HD + D reaction: comparison between experiment and theoretical predictions. J. Chem. Phys. 115, 4534–4545 (2001)
Althorpe, S. C. et al. Observation and interpretation of a time-delayed mechanism in the hydrogen exchange reaction. Nature 416, 67–70 (2002)
Aoiz, F. J., Herrero, V. J. & Saez Rabanos, V. Quasiclassical state to state reaction cross sections for D + H2(ν = 0,j = 0) → HD(ν′,j′) + H. Formation and characteristics of short-lived collision complexes. J. Chem. Phys. 97, 7423–7436 (1992)
Allison, T. C., Friedman, R. S., Kaufman, D. J. & Truhlar, D. G. Analysis of the resonance in H + D2 → HD(v′ = 3) + D. Chem. Phys. Lett. 327, 439–445 (2000)
Buntin, S. A., Giese, C. F. & Gentry, W. R. State-resolved differential cross sections for the reaction D + H2 → HD + H. J. Chem. Phys. 87, 1443–1445 (1987)
Kliner, D. A., Adelman, D. E. & Zare, R. N. Comparison of experimental and theoretical integral cross sections for D + H2(ν = 1,j = 1) → HD(ν′ = 1,j′) + H. J. Chem. Phys. 95, 1648–1662 (1991)
Kitsopoulos, T. N., Buntine, M. A., Balwin, D. P., Zare, R. N. & Chandler, D. W. Reaction product imaging: The H + D2 reaction. Science 260, 1605–1610 (1993)
Schnieder, L. et al. Experimental studies and theoretical predictions for the H + D2 → HD + D reaction. Science 269, 207–210 (1995)
Schnieder, L., Seekamp-Rahn, K., Wrede, E. & Welge, K. H. Experimental determination of quantum state resolved differential cross sections for the hydrogen exchange reaction H + D2 → HD + D. J. Chem. Phys. 107, 6175–6195 (1997)
Zhang, J. Z. H. & Miller, W. H. Quantum reactive scattering via the S-matrix version of the Kohn variational principle: Differential and integral cross sections for D + H2 → HD + H. J. Chem. Phys. 91, 1528–1547 (1991)
Zhao, M., Truhlar, D. G., Schwenke, D. W. & Kouri, D. J. Effect of rotational excitation on state-to-state differential cross sections: deuterium atom + hydrogen → hydrogen deuteride + hydrogen atom. J. Phys. Chem. 94, 7074–7090 (1990)
Zhao, M. et al. Spectroscopic analysis of transition state energy levels: Bending–rotational spectrum and lifetime analysis of H3 quasibound states. J. Chem. Phys. 91, 5302–5309 (1989)
D'Mello, M. J., Manolopoulos, D. E. & Wyatt, R. E. Quantum dynamics of the H + D2 → D + HD reaction: Comparison with experiment. J. Chem. Phys. 94, 5985–5993 (1991)
Skouteris, D. M., Castillo, J. F. & Manolopoulos, D. E. ABC: a quantum reactive scattering program. Comput. Phys. Commun. 133, 128–135 (2000)
Chao, S. D. & Skodje, R. T. The search for resonance signatures in H + D2 reaction dynamics. Chem. Phys. Lett. 336, 364–370 (2001)
Schnieder, L. et al. Photodissociation dynamics of H2S at 121.6 nm and a determination of the potential energy function of SH(A2Σ+). J. Chem. Phys. 92, 7027–7037 (1990)
Boothroyd, A. I., Keogh, W. J., Martin, P. G. & Peterson, M. R. A refined H3 potential energy surface. J. Chem. Phys. 104, 7139–7152 (1996)
Goldberger, M. L. & Watson, K. M. Collision Theory (Wiley, New York, 1964)
Cuccaro, S. A., Hipes, P. G. & Kuppermann, A. Symmetry analysis of accurate H + H2 resonances for low partial waves. Chem. Phys. Lett. 157, 440–446 (1989)
Skodje, R. T., Sadeghi, R., Koppel, H. & Krause, J. L. Spectral quantization of transition state dynamics for the three-dimensional H + H2 reaction. J. Chem. Phys. 101, 1725–1729 (1994)
Chatfield, D. C., Mielke, S. L., Allison, T. C. & Truhlar, D. G. Quantized dynamical bottlenecks and transition state control of the reaction of D with H2: Effect of varying the total angular momentum. J. Chem. Phys. 112, 8387–8408 (2000)
Sadeghi, R. & Skodje, R. T. Barriers, thresholds, and resonances: Spectral quantization of the transition state for the collinear D + H2 reaction. J. Chem. Phys. 102, 193–213 (1995)
Chatfield, D. C., Friedman, R. S., Schwenke, D. W. & Truhlar, D. G. Control of chemical reactivity by quantized transition states. J. Phys. Chem. 96, 2414–2421 (1992)
Harich, S. A., Dai, D., Yang, X., Chao, S. D. & Skodje, R. T. State-to-state dynamics of H + HD → H2 + D at 0.5 eV: A combined theoretical and experimental study. J. Chem. Phys. 116, 4769–4772 (2002)
Liu, X., Lin, J. J., Harich, S. A., Schatz, G. C. & Yang, X. A quantum state-resolved insertion reaction: O(1D) + H2(j = 0) → OH(2Π,ν,N) + H(2S). Science 289, 1536–1538 (2000)
Kuppermann, A., Schatz, G. C. & Baer, M. Quantum mechanical reactive scattering for planar atom plus diatom systems. I. Theory. J. Chem. Phys. 65, 4596–4623 (1976)
Acknowledgements
We thank K. Liu and Y.T. Lee for many helpful discussions. The experimental work was supported mainly by the National Science Council and Academia Sinica of Taiwan, and the theoretical effort was supported by the National Science Foundation of USA, and by the Ministry of Education, Culture, Sports, Science and Technology of Japan. D.D. and X.Y. also acknowledge support for this work at DICP by the Ministry of Science & Technology of China.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Harich, S., Dai, D., Wang, C. et al. Forward scattering due to slow-down of the intermediate in the H + HD → D + H2 reaction. Nature 419, 281–284 (2002). https://doi.org/10.1038/nature01068
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01068
This article is cited by
-
Observation of geometric phase effect through backward angular oscillations in the H + HD → H2 + D reaction
Nature Communications (2024)
-
Tunnelling measured in a very slow ion–molecule reaction
Nature (2023)
-
Molecular collisions: From near-cold to ultra-cold
Frontiers of Physics (2021)
-
Observation of the geometric phase effect in the H+HD→H2+D reaction below the conical intersection
Nature Communications (2020)
-
Direct observation of forward-scattering oscillations in the H+HD→H2+D reaction
Nature Chemistry (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.