Abstract
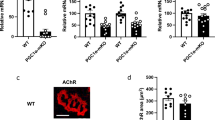
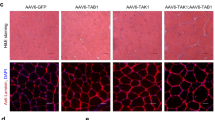
The biochemical basis for the regulation of fibre-type determination in skeletal muscle is not well understood. In addition to the expression of particular myofibrillar proteins, type I (slow-twitch) fibres are much higher in mitochondrial content and are more dependent on oxidative metabolism than type II (fast-twitch) fibres1. We have previously identified a transcriptional co-activator, peroxisome-proliferator-activated receptor-γ co-activator-1 (PGC-1α), which is expressed in several tissues including brown fat and skeletal muscle, and that activates mitochondrial biogenesis and oxidative metabolism2,3,4. We show here that PGC-1α is expressed preferentially in muscle enriched in type I fibres. When PGC-1α is expressed at physiological levels in transgenic mice driven by a muscle creatine kinase (MCK) promoter, a fibre type conversion is observed: muscles normally rich in type II fibres are redder and activate genes of mitochondrial oxidative metabolism. Notably, putative type II muscles from PGC-1α transgenic mice also express proteins characteristic of type I fibres, such as troponin I (slow) and myoglobin, and show a much greater resistance to electrically stimulated fatigue. Using fibre-type-specific promoters, we show in cultured muscle cells that PGC-1α activates transcription in cooperation with Mef2 proteins and serves as a target for calcineurin signalling, which has been implicated in slow fibre gene expression. These data indicate that PGC-1α is a principal factor regulating muscle fibre type determination.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Berchtold, M. W., Brinkmeier, H. & Muntener, M. Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol. Rev. 80, 1215–1265 (2000)
Puigserver, P. et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 (1998)
Wu, Z. et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98, 115–124 (1999)
Vega, R. B., Huss, J. M. & Kelly, D. P. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor α in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol. Cell. Biol. 20, 1868–1876 (2000)
Booth, F. W. & Thomason, D. B. Molecular and cellular adaptation of muscle in response to exercise: perspectives of various models. Physiol. Rev. 71, 541–585 (1991)
Hood, D. A. Contractile activity-induced mitochondrial biogenesis in skeletal muscle. J. Appl. Physiol. 90, 1137–1157 (2001)
Gollnick, P. D. et al. Enzyme activity and fibre composition in skeletal muscle of untrained and trained men. J. Appl. Physiol. 33, 312–319 (1972)
Jarvis, J. C. et al. Fast-to-slow transformation in stimulated rat muscle. Muscle Nerve 19, 1469–1475 (1996)
Chin, E. R. et al. A calcineurin-dependent transcriptional pathway controls skeletal muscle fibre type. Genes Dev. 12, 2499–2509 (1998)
Naya, F. J. et al. Stimulation of slow skeletal muscle fibre gene expression by calcineurin in vivo. J. Biol. Chem. 275, 4545–4548 (2000)
Bigard, X. et al. Calcineurin co-regulates contractile and metabolic components of slow muscle phenotype. J. Biol. Chem. 275, 19653–19660 (2000)
Olson, E. N. & Williams, R. S. Remodeling muscles with calcineurin. Bioassays 22, 510–519 (2000)
Lehman, J. J. et al. Peroxisome proliferator-activated receptor γ coactivator-1 promotes cardiac mitochondrial biogenesis. J. Clin. Invest. 106, 847–856 (2000)
Yoon, J. C. et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413, 131–138 (2001)
Esterbauer, H. et al. Human peroxisome proliferator activated receptor gamma coactivator 1 (PPARGC1) gene: cDNA sequence, genomic organization, chromosomal localization, and tissue expression. Genomics 62, 98–102 (1999)
Lin, J. et al. PGC-1β: a novel PGC-1 related transcription coactivator associated with host cell factor. J. Biol. Chem. 277, 1645–1648 (2002)
Johnson, J. E., Wold, B. J. & Hauschka, S. D. Muscle creatine kinase sequence elements regulating skeletal and cardiac muscle expression in transgenic mice. Mol. Cell. Biol. 9, 3393–3399 (1989)
Ogilvie, R. W. & Feeback, D. L. A metachromatic dye-ATPase method for the simultaneous identification of skeletal muscle fibre types I, IIA, IIB and IIC. Stain Technol. 65, 231–241 (1990)
Grange, R. W. et al. Functional and molecular adaptations in skeletal muscle of myoglobin-mutant mice. Am. J. Physiol. Cell Physiol. 281, C1487–C1494 (2001)
Wu, H. et al. MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fibre type. EMBO J. 19, 1963–1973 (2000)
Wu, H. et al. Activation of MEF2 by muscle activity is mediated through a calcineurin-dependent pathway. EMBO J. 20, 6414–6423 (2001)
Calvo, S. et al. Fibre-type-specific transcription of the troponin I slow gene is regulated by multiple elements. Mol. Cell. Biol. 19, 515–525 (1999)
Black, B. L. & Olson, E. N. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu. Rev. Cell Dev. Biol. 14, 167–196 (1998)
Delling, U. et al. A calcineurin-NFATc3-dependent pathway regulates skeletal muscle differentiation and slow myosin heavy-chain expression. Mol. Cell. Biol. 20, 6600–6611 (2000)
Michael, L. F. et al. Restoration of insulin-sensitive glucose transporter (Glut4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc. Natl Acad. Sci. USA 98, 3820–3825 (2001)
Nakayama, M. et al. Common core sequences are found in skeletal muscle slow- and fast-fibre-type-specific regulatory elements. Mol. Cell. Biol. 16, 2408–2417 (1996)
Goto, M. et al. cDNA cloning and mRNA analysis of PGC-1 in epitrochlearis muscle in swimming-exercised rats. Biochem. Biophys. Res. Comm. 274, 350–354 (2000)
Acknowledgements
We thank R. Sanders Williams and A. Buonanno for providing reporter constructs, and J. Lawitts for generating transgenic mice. This work was supported by grants from the NIH to B.M.S, B.B.L and E.N.O. J.L. was supported by a postdoctoral fellowship from the American Heart Association.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Lin, J., Wu, H., Tarr, P. et al. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature 418, 797–801 (2002). https://doi.org/10.1038/nature00904
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature00904
This article is cited by
-
Vitamin A regulates mitochondrial biogenesis and function through p38 MAPK-PGC-1α signaling pathway and alters the muscle fiber composition of sheep
Journal of Animal Science and Biotechnology (2024)
-
Peroxisome proliferator-activated receptor γ coactivator 1α regulates downstream of tyrosine kinase-7 (Dok-7) expression important for neuromuscular junction formation
Scientific Reports (2024)
-
Dietary oleic acid intake increases the proportion of type 1 and 2X muscle fibers in mice
Scientific Reports (2024)
-
Exercise-Regulated Mitochondrial and Nuclear Signalling Networks in Skeletal Muscle
Sports Medicine (2024)
-
Tropomyosin 3 (TPM3) function in skeletal muscle and in myopathy
Skeletal Muscle (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.