Abstract
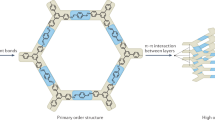
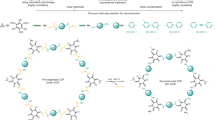
Covalent organic frameworks (COFs) are a class of crystalline porous polymer that allows the atomically precise integration of organic units into extended structures with periodic skeletons and ordered nanopores. One important feature of COFs is that they are designable; that is, the geometry and dimensions of the building blocks can be controlled to direct the topological evolution of structural periodicity. The diversity of building blocks and covalent linkage topology schemes make COFs an emerging materials platform for structural control and functional design. Indeed, COF architectures offer confined molecular spaces for the interplay of photons, excitons, electrons, holes, ions and guest molecules, thereby exhibiting unique properties and functions. In this Review, we summarize the major progress in the field of COFs and recent achievements in developing new design principles and synthetic strategies. We highlight cutting-edge functional designs and identify fundamental issues that need to be addressed in conjunction with future research directions from chemistry, physics and materials perspectives.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Feng, X., Ding, X. & Jiang, D. Covalent organic frameworks. Chem. Soc. Rev. 41, 6010–6022 (2012).
Ding, S.-Y. & Wang, W. Covalent organic frameworks (COFs): from design to applications. Chem. Soc. Rev. 42, 548–568 (2013).
Dogru, M. & Bein, T. On the road towards electroactive covalent organic frameworks. Chem. Commun. 50, 5531–5546 (2014).
Waller, P. J., Gándara, F. & Yaghi, O. M. Chemistry of covalent organic frameworks. Acc. Chem. Res. 48, 3053–3063 (2015).
Côté, A. P. et al. Porous, crystalline, covalent organic frameworks. Science 310, 1166–1170 (2005). The first example of COFs was described in this study using a boronate ester linkage.
El-Kaderi, H. M. et al. Designed synthesis of 3D covalent organic frameworks. Science 316, 268–272 (2007).
Wan, S., Guo, J., Kim, J., Ihee, H. & Jiang, D. A belt-shaped, blue luminescent, and semiconducting covalent organic framework. Angew. Chem. Int. Ed. 47, 8826–8830 (2008). The first example of semiconducting COFs.
Wan, S., Guo, J., Kim, J., Ihee, H. & Jiang, D. A photoconductive covalent organic framework: self-condensed arene cubes composed of eclipsed 2D polypyrene sheets for photocurrent generation. Angew. Chem. Int. Ed. 48, 5439–5442 (2009).
Wan, S. et al. Covalent organic frameworks with high charge carrier mobility. Chem. Mater. 23, 4094–4097 (2011).
Ding, X. et al. An n-channel two-dimensional covalent organic framework. J. Am. Chem. Soc. 133, 14510–14513 (2011).
Feng, X. et al. High-rate charge-carrier transport in porphyrin covalent organic frameworks: switching from hole to electron to ambipolar conduction. Angew. Chem. Int. Ed. 51, 2618–2622 (2012). Porphyrin-based COFs were demonstrated to conduct charge carriers.
Feng, X. et al. An ambipolar conducting covalent organic framework with self-sorted and periodic electron donor–acceptor ordering. Adv. Mater. 24, 3026–3031 (2012).
Guo, J. et al. Conjugated organic framework with three-dimensionally ordered stable structure and delocalized π clouds. Nat. Commun. 4, 2736 (2013).
Dogru, M. et al. A photoconductive thienothiophene-based covalent organic framework showing charge transfer towards included fullerene. Angew. Chem. Int. Ed. 52, 2920–2924 (2013). A photoinduced electron-transferring COF was exemplified to show potential use in solar cells.
Jin, S. et al. Two-dimensional tetrathiafulvalene covalent organic frameworks: towards latticed conductive organic salts. Chem. Eur. J. 20, 14608–14613 (2014).
Ding, H. et al. A tetrathiafulvalene-based electroactive covalent organic framework. Chem. Eur. J. 20, 14614–14618 (2014).
Cai, S.-L. et al. Tunable electrical conductivity in oriented thin films of tetrathiafulvalene-based covalent organic framework. Chem. Sci. 5, 4693–4700 (2014).
Chen, L. et al. Photoelectric covalent organic frameworks: converting open lattices into ordered donor–acceptor heterojunctions. J. Am. Chem. Soc. 136, 9806–9809 (2014).
Duhovic´, S. & Dincaˇ, M. Synthesis and electrical properties of covalent organic frameworks with heavy chalcogens. Chem. Mater. 27, 5487–5490 (2015).
Dalapati, S. et al. Rational design of crystalline supermicroporous covalent organic frameworks with triangular topologies. Nat. Commun. 6, 7786 (2015).
Feldblyum, J. I. et al. Few-layer, large-area, 2D covalent organic framework semiconductor thin films. Chem. Commun. 51, 13894–13897 (2015).
Li, Z. et al. A 2D azine-linked covalent organic framework for gas storage applications. Chem. Commun. 50, 13825–13828 (2014).
Huang, N., Krishna, R. & Jiang, D. Tailor-made pore surface engineering in covalent organic frameworks: systematic functionalization for performance screening. J. Am. Chem. Soc. 137, 7079–7082 (2015).
Huang, N., Chen, X., Krishna, R. & Jiang, D. Two-dimensional covalent organic frameworks for carbon dioxide capture through channel-wall functionalization. Angew. Chem. Int. Ed. 54, 2986–2990 (2015).
Chandra, S. et al. Phosphoric acid loaded azo (–N=N–) based covalent organic framework for proton conduction. J. Am. Chem. Soc. 136, 6570–6573 (2014).
Xu, H., Tao, S. & Jiang, D. Proton conduction in crystalline and porous covalent organic frameworks. Nat. Mater. 15, 722–726 (2016).
Ma, H. et al. Cationic covalent organic frameworks: a simple platform of anionic exchange for porosity tuning and proton conduction. J. Am. Chem. Soc. 138, 5897–5903 (2016).
Chandra, S. et al. Interplaying intrinsic and extrinsic proton conductivities in covalent organic frameworks. Chem. Mater. 28, 1489–1494 (2016).
Dalapati, S. et al. An azine-linked covalent organic framework. J. Am. Chem. Soc. 135, 17310–17313 (2013). Azine linkage was developed for the synthesis of COFs.
Ding, S.-Y. et al. Thioether-based fluorescent covalent organic framework for selective detection and facile removal of mercury(II). J. Am. Chem. Soc. 138, 3031–3037 (2016).
Dalapati, S., Jin, E., Addicoat, M., Heine, T. & Jiang, D. Highly emissive covalent organic frameworks. J. Am. Chem. Soc. 138, 5797–5800 (2016).
Ding, S.-Y. et al. Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in Suzuki–Miyaura coupling reaction. J. Am. Chem. Soc. 133, 19816–19822 (2011). First demonstration of catalytic COFs.
Nagai, A. et al. A squaraine-linked mesoporous covalent organic framework. Angew. Chem. Int. Ed. 52, 3770–3774 (2013).
Pachfule, P. et al. Multifunctional and robust covalent organic framework–nanoparticle hybrids. J. Mater. Chem. A 2, 7944–7952 (2014).
Shinde, D. B., Kandambeth, S., Pachfule, P., Kumar, R. R. & Banerjee, R. Bifunctional covalent organic frameworks with two dimensional organocatalytic micropores. Chem. Commun. 51, 310–313 (2015).
Xu, H., Gao, J. & Jiang, D. Stable, crystalline, porous, covalent organic frameworks as a platform for chiral organocatalysts. Nat. Chem. 7, 905–912 (2015).
Lin, S. et al. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 349, 1208–1213 (2015).
Thote, J. et al. A covalent organic framework–cadmium sulfide hybrid as a prototype photocatalyst for visible-light-driven hydrogen production. Chem. Eur. J. 20, 15961–15965 (2014).
Stegbauer, L., Schwinghammer, K. & Lotsch, B. V. A hydrazone-based covalent organic framework for photocatalytic hydrogen production. Chem. Sci. 5, 2789–2793 (2014).
Vyas, V. S. et al. A tunable azine covalent organic framework platform for visible light-induced hydrogen generation. Nat. Commun. 6, 8508 (2015).
DeBlase, C. R., Silberstein, K. E., Truong, T.-T., Abruña, H. D. & Dichtel, W. R. β-Ketoenamine-linked covalent organic frameworks capable of pseudocapacitive energy storage. J. Am. Chem. Soc. 135, 16821–16824 (2013).
Xu, F. et al. Electrochemically active, crystalline, mesoporous covalent organic frameworks on carbon nanotubes for synergistic lithium-ion battery energy storage. Sci. Rep. 5, 8225 (2015).
Xu, F. et al. Radical covalent organic frameworks: a general strategy to immobilize open-accessible polyradicals for high-performance capacitive energy storage. Angew. Chem. Int. Ed. 54, 6814–6818 (2015).
Tilford, R. W., Gemmill, W. R., zur Loye, H.-C. & Lavigne, J. J. Facile synthesis of a highly crystalline, covalently linked porous boronate network. Chem. Mater. 18, 5296–5301 (2006).
Côté, A. P., El-Kaderi, H. M., Furukawa, H., Hunt, J. R. & Yaghi, O. M. Reticular synthesis of microporous and mesoporous 2D covalent organic frameworks. J. Am. Chem. Soc. 129, 12914–12915 (2007).
Tilford, R. W., Mugavero, S. J., Pellechia, P. J. & Lavigne, J. J. Tailoring microporosity in covalent organic frameworks. Adv. Mater. 20, 2741–2746 (2008).
Dogru, M., Sonnauer, A., Gavryushin, A., Knochel, P. & Bein, T. A covalent organic framework with 4 nm open pores. Chem. Commun. 47, 1707–1709 (2011).
Spitler, E. L. et al. A 2D covalent organic framework with 4.7-nm pores and insight into its interlayer stacking. J. Am. Chem. Soc. 133, 19416–19421 (2011).
Yu, J.-T., Chen, Z., Sun, J., Huang, Z.-T. & Zheng, Q.-Y. Cyclotricatechylene based porous crystalline material: synthesis and applications in gas storage. J. Mater. Chem. 22, 5369–5373 (2012).
Kahveci, Z., Islamoglu, T., Shar, G. A., Ding, R. & El-Kaderi, H. M. Targeted synthesis of a mesoporous triptycene-derived covalent organic framework. CrystEngComm 15, 1524–1527 (2013).
Bertrand, G. H. V., Michaelis, V. K., Ong, T.-C., Griffin, R. G. & Dincaˇ, M. Thiophene-based covalent organic frameworks. Proc. Natl Acad. Sci. USA 110, 4923–4928 (2013).
Feng, X., Dong, Y. & Jiang, D. Star-shaped two-dimensional covalent organic frameworks. CrystEngComm 15, 1508–1511 (2013).
Zhang, J. et al. A novel azobenzene covalent organic framework. CrystEngComm 16, 6547–6551 (2014).
Fang, Q. et al. Designed synthesis of large-pore crystalline polyimide covalent organic frameworks. Nat. Commun. 5, 4503 (2014).
Song, J.-R., Sun, J., Liu, J., Huang, Z.-T. & Zheng, Q.-Y. Thermally/hydrolytically stable covalent organic frameworks from a rigid macrocyclic host. Chem. Commun. 50, 788–791 (2014).
Das, G. et al. Chemical sensing in two dimensional porous covalent organic nanosheets. Chem. Sci. 6, 3931–3939 (2015).
Yang, H. et al. Mesoporous 2D covalent organic frameworks based on shape-persistent arylene-ethynylene macrocycles. Chem. Sci. 6, 4049–4053 (2015).
Biswal, B. P. et al. Pore surface engineering in porous, chemically stable covalent organic frameworks for water adsorption. J. Mater. Chem. A 3, 23664–23669 (2015).
Baldwin, L. A., Crowe, J. W., Shannon, M. D., Jaroniec, C. P. & McGrier, P. L. 2D covalent organic frameworks with alternating triangular and hexagonal pores. Chem. Mater. 27, 6169–6172 (2015).
Lin, S. et al. A triazine-based covalent organic framework/palladium hybrid for one-pot silicon-based cross-coupling of silanes and aryl iodides. RSC Adv. 5, 41017–41024 (2015).
Li, Z., Zhang, Y., Xia, H., Mu, Y. & Liu, X. A robust and luminescent covalent organic framework as a highly sensitive and selective sensor for the detection of Cu2+ ions. Chem. Commun. 52, 6613–6616 (2016).
Zhen, J. et al. Preparation of a series of aCTV-based covalent organic frameworks and substituent effects on their properties. CrystEngComm 18, 1039–1045 (2016).
Lohse, M. S. et al. Sequential pore wall modification in a covalent organic framework for application in lactic acid adsorption. Chem. Mater. 28, 626–631 (2016).
Li, Z.-J., Ding, S.-Y., Xue, H.-D., Cao, W. & Wang, W. Synthesis of –C=N– linked covalent organic frameworks via the direct condensation of acetals and amines. Chem. Commun. 52, 7217–7220 (2016).
Lohse, M. S. et al. From benzodithiophene to diethoxy-benzodithiophene covalent organic frameworks — structural investigations. CrystEngComm 18, 4295–4302 (2016).
Kuhn, P., Antonietti, M. & Thomas, A. Porous, covalent triazine-based frameworks prepared by ionothermal synthesis. Angew. Chem. Int. Ed. 47, 3450–3453 (2008).
Jackson, K. T., Reich, T. E. & El-Kaderi, H. M. Targeted synthesis of a porous borazine-linked covalent organic framework. Chem. Commun. 48, 8823–8825 (2012).
Xie, Y.-F., Ding, S.-Y., Liu, J.-M., Wang, W. & Zheng, Q.-Y. Triazatruxene based covalent organic framework and its quick-response fluorescence-on nature towards electron rich arenes. J. Mater. Chem. C 3, 10066–10069 (2015).
de la Peña Ruigómez, A. et al. Direct on-surface patterning of a crystalline laminar covalent organic framework synthesized at room temperature. Chem. Eur. J. 21, 10666–10670 (2015).
Kaleeswaran, D., Vishnoi, P. & Murugavel, R. [3 + 3] Imine and β-ketoenamine tethered fluorescent covalent-organic frameworks for CO2 uptake and nitroaromatic sensing. J. Mater. Chem. C 3, 7159–7171 (2015).
Xu, L. et al. Highly crystalline covalent organic frameworks from flexible building blocks. Chem. Commun. 52, 4706–4709 (2016).
Spitler, E. L. & Dichtel, W. R. Lewis acid-catalysed formation of two-dimensional phthalocyanine covalent organic frameworks. Nat. Chem. 2, 672–677 (2010).
Feng, X., Chen, L., Dong, Y. & Jiang, D. Porphyrin-based two-dimensional covalent organic frameworks: synchronized synthetic control of macroscopic structures and pore parameters. Chem. Commun. 47, 1979–1981 (2011).
Ding, X. et al. Synthesis of metallophthalocyanine covalent organic frameworks that exhibit high carrier mobility and photoconductivity. Angew. Chem. Int. Ed. 50, 1289–1293 (2011).
Ding, X. et al. Conducting metallophthalocyanine 2D covalent organic frameworks: the role of central metals in controlling π-electronic functions. Chem. Commun. 48, 8952–8954 (2012).
Neti, V. S. P. K. et al. Synthesis of a phthalocyanine 2D covalent organic framework. CrystEngComm 15, 7157–7160 (2013).
Neti, V. S. P. K., Wu, X., Deng, S. & Echegoyen, L. Synthesis of a phthalocyanine and porphyrin 2D covalent organic framework. CrystEngComm 15, 6892–6895 (2013).
Chen, X., Gao, J. & Jiang, D. Designed synthesis of porphyrin-based two-dimensional covalent organic frameworks with highly ordered structures. Chem. Lett. 44, 1257–1259 (2015).
Chen, X. et al. Towards covalent organic frameworks with predesignable and aligned open docking sites. Chem. Commun. 50, 6161–6163 (2014).
Wu, Y., Xu, H., Chen, X., Gao, J. & Jiang, D. A π-electronic covalent organic framework catalyst: π-walls as catalytic beds for Diels–Alder reactions under ambient conditions. Chem. Commun. 51, 10096–10098 (2015).
Zhou, T.-Y., Xu, S.-Q., Wen, Q., Pang, Z.-F. & Zhao, X. One-step construction of two different kinds of pores in a 2D covalent organic framework. J. Am. Chem. Soc. 136, 15885–15888 (2014).
Alahakoon, S. B. et al. An azine-linked hexaphenylbenzene based covalent organic framework. Chem. Commun. 52, 2843–2845 (2016).
Xu, S.-Q., Zhan, T.-G., Wen, Q., Pang, Z.-F. & Zhao, X. Diversity of covalent organic frameworks (COFs): a 2D COF containing two kinds of triangular micropores of different sizes. ACS Macro Lett. 5, 99–102 (2016).
Pang, Z.-F. et al. Construction of covalent organic frameworks bearing three different kinds of pores through the heterostructural mixed linker strategy. J. Am. Chem. Soc. 138, 4710–4713 (2016).
Zhu, Y., Wan, S., Jin, Y. & Zhang, W. Desymmetrized vertex design for the synthesis of covalent organic frameworks with periodically heterogeneous pore structures. J. Am. Chem. Soc. 137, 13772–13775 (2015).
Spitler, E. L., Giovino, M. R., White, S. L. & Dichtel, W. R. A mechanistic study of Lewis acid-catalyzed covalent organic framework formation. Chem. Sci. 2, 1588–1593 (2011).
Uribe-Romo, F. J. et al. A crystalline imine-linked 3D porous covalent organic framework. J. Am. Chem. Soc. 131, 4570–4571 (2009). Imine linkage was developed for the synthesis of COFs.
Uribe-Romo, F. J., Doonan, C. J., Furukawa, H., Oisaki, K. & Yaghi, O. M. Crystalline covalent organic frameworks with hydrazone linkages. J. Am. Chem. Soc. 133, 11478–11481 (2011).
Zeng, Y. et al. Covalent organic frameworks formed with two types of covalent bonds based on orthogonal reactions. J. Am. Chem. Soc. 137, 1020–1023 (2015).
Chen, X. et al. Designed synthesis of double-stage two-dimensional covalent organic frameworks. Sci. Rep. 5, 14650 (2015).
Zhang, W., Jiang, P., Wang, Y., Zhang, J. & Zhang, P. Bottom-up approach to engineer two covalent porphyrinic frameworks as effective catalysts for selective oxidation. Catal. Sci. Technol. 5, 101–104 (2015).
Du, Y. et al. Ionic covalent organic frameworks with spiroborate linkage. Angew. Chem. Int. Ed. 55, 1737–1741 (2016).
Nath, B. et al. A new azodioxy-linked porphyrin-based semiconductive covalent organic framework with I2 doping-enhanced photoconductivity. CrystEngComm 18, 4259–4263 (2016).
Bojdys, M. J., Jeromenok, J., Thomas, A. & Antonietti, M. Rational extension of the family of layered, covalent, triazine-based frameworks with regular porosity. Adv. Mater. 22, 2202–2205 (2010).
Kuecken, S., Schmidt, J., Zhi, L. & Thomas, A. Conversion of amorphous polymer networks to covalent organic frameworks under ionothermal conditions: a facile synthesis route for covalent triazine frameworks. J. Mater. Chem. A 3, 24422–24427 (2015).
Campbell, N. L., Clowes, R., Ritchie, L. K. & Cooper, A. I. Rapid microwave synthesis and purification of porous covalent organic frameworks. Chem. Mater. 21, 204–206 (2009).
Ren, S. et al. Porous, fluorescent, covalent triazine-based frameworks via room-temperature and microwave-assisted synthesis. Adv. Mater. 24, 2357–2361 (2012).
Dogru, M. et al. Facile synthesis of a mesoporous benzothiadiazole-COF based on a transesterification process. CrystEngComm 15, 1500–1502 (2013).
Wei, H. et al. The microwave-assisted solvothermal synthesis of a crystalline two-dimensional covalent organic framework with high CO2 capacity. Chem. Commun. 51, 12178–12181 (2015).
Biswal, B. P. et al. Mechanochemical synthesis of chemically stable isoreticular covalent organic frameworks. J. Am. Chem. Soc. 135, 5328–5331 (2013).
Chandra, S. et al. Chemically stable multilayered covalent organic nanosheets from covalent organic frameworks via mechanical delamination. J. Am. Chem. Soc. 135, 17853–17861 (2013).
Das, G., Balaji Shinde, D., Kandambeth, S., Biswal, B. P. & Banerjee, R. Mechanosynthesis of imine, ß-ketoenamine, and hydrogen-bonded imine-linked covalent organic frameworks using liquid-assisted grinding. Chem. Commun. 50, 12615–12618 (2014).
Blunt, M. O., Russell, J. C., Champness, N. R. & Beton, P. H. Templating molecular adsorption using a covalent organic framework. Chem. Commun. 46, 7157–7159 (2010).
Marele, A. C. et al. Formation of a surface covalent organic framework based on polyester condensation. Chem. Commun. 48, 6779–6781 (2012).
Gutzler, R. et al. Surface mediated synthesis of 2D covalent organic frameworks: 1,3,5-tris(4-bromophenyl)benzene on graphite(001), Cu(111), and Ag(110). Chem. Commun. 2009, 4456–4458 (2009).
Zwaneveld, N. A. A. et al. Organized formation of 2D extended covalent organic frameworks at surfaces. J. Am. Chem. Soc. 130, 6678–6679 (2008).
Larrea, C. R. & Baddeley, C. J. Fabrication of a high-quality, porous, surface-confined covalent organic framework on a reactive metal surface. ChemPhysChem 17, 971–975 (2016).
Hao, D. et al. Fabrication of a COF-5 membrane on a functionalized α-Al2O3 ceramic support using a microwave irradiation method. Chem. Commun. 50, 1462–1464 (2014).
Colson, J. W. et al. Oriented 2D covalent organic framework thin films on single-layer graphene. Science 332, 228–231 (2011). A method to prepare COF films on graphene was demonstrated in this study.
Spitler, E. L. et al. Lattice expansion of highly oriented 2D phthalocyanine covalent organic framework films. Angew. Chem. Int. Ed. 51, 2623–2627 (2012).
Xu, L. et al. Surface-confined single-layer covalent organic framework on single-layer graphene grown on copper foil. Angew. Chem. Int. Ed. 53, 9564–9568 (2014).
Zha, Z. et al. 3D graphene functionalized by covalent organic framework thin film as capacitive electrode in alkaline media. ACS Appl. Mater. Interfaces 7, 17837–17843 (2015).
Colson, J. W., Mann, J. A., DeBlase, C. R. & Dichtel, W. R. Patterned growth of oriented 2D covalent organic framework thin films on single-layer graphene. J. Polym. Sci. Part A: Polym. Chem. 53, 378–384 (2015).
Yue, J.-Y., Liu, X.-H., Sun, B. & Wang, D. The on-surface synthesis of imine-based covalent organic frameworks with non-aromatic linkage. Chem. Commun. 51, 14318–14321 (2015).
Medina, D. D. et al. Oriented thin films of a benzodithiophene covalent organic framework. ACS Nano 8, 4042–4052 (2014).
Gou, X. et al. Preparation and engineering of oriented 2D covalent organic framework thin films. RSC Adv. 6, 39198–39203 (2016).
Chen, Y. et al. Surface growth of highly oriented covalent organic framework thin film with enhanced photoresponse speed. RSC Adv. 5, 92573–92576 (2015).
Medina, D. D. et al. Room temperature synthesis of covalent-organic framework films through vapor-assisted conversion. J. Am. Chem. Soc. 137, 1016–1019 (2015).
Hunt, J. R., Doonan, C. J., LeVangie, J. D., Côté, A. P. & Yaghi, O. M. Reticular synthesis of covalent organic borosilicate frameworks. J. Am. Chem. Soc. 130, 11872–11873 (2008).
Bunck, D. N. & Dichtel, W. R. Internal functionalization of three-dimensional covalent organic frameworks. Angew. Chem. Int. Ed. 51, 1885–1889 (2012).
Bunck, D. N. & Dichtel, W. R. Postsynthetic functionalization of 3D covalent organic frameworks. Chem. Commun. 49, 2457–2459 (2013).
Zhang, Y.-B. et al. Single-crystal structure of a covalent organic framework. J. Am. Chem. Soc. 135, 16336–16339 (2013).
Fang, Q. et al. 3D Microporous base-functionalized covalent organic frameworks for size-selective catalysis. Angew. Chem. Int. Ed. 53, 2878–2882 (2014).
Fang, Q. et al. 3D porous crystalline polyimide covalent organic frameworks for drug delivery. J. Am. Chem. Soc. 137, 8352–8355 (2015).
Lin, G., Ding, H., Yuan, D., Wang, B. & Wang, C. A pyrene-based, fluorescent three-dimensional covalent organic framework. J. Am. Chem. Soc. 138, 3302–3305 (2016).
Liu, Y. et al. Weaving of organic threads into a crystalline covalent organic framework. Science 351, 365–369 (2016).
Chen, X., Addicoat, M., Irle, S., Nagai, A. & Jiang, D. Control of crystallinity and porosity of covalent organic frameworks by managing interlayer interactions based on self-complementary π-electronic force. J. Am. Chem. Soc. 135, 546–549 (2013).
Kandambeth, S. et al. Enhancement of chemical stability and crystallinity in porphyrin-containing covalent organic frameworks by intramolecular hydrogen bonds. Angew. Chem. Int. Ed. 52, 13052–13056 (2013).
Chen, X. et al. Locking covalent organic frameworks with hydrogen bonds: general and remarkable effects on crystalline structure, physical properties, and photochemical activity. J. Am. Chem. Soc. 137, 3241–3247 (2015).
Kandambeth, S. et al. Construction of crystalline 2D covalent organic frameworks with remarkable chemical (acid/base) stability via a combined reversible and irreversible route. J. Am. Chem. Soc. 134, 19524–19527 (2012). A unique imine-based COF (TpPa-1) with enol-to-keto tautomerization-induced structural stabilization.
Ascherl, L. et al. Molecular docking sites designed for the generation of highly crystalline covalent organic frameworks. Nat. Chem. 8, 310–316 (2016).
Yu, S.-B. et al. A polycationic covalent organic framework: a robust adsorbent for anionic dye pollutants. Polym. Chem. 7, 3392–3397 (2016).
Jin, S. et al. Large pore donor–acceptor covalent organic frameworks. Chem. Sci. 4, 4505–4511 (2013).
Furukawa, H. & Yaghi, O. M. Storage of hydrogen, methane, and carbon dioxide in highly porous covalent organic frameworks for clean energy applications. J. Am. Chem. Soc. 131, 8875–8883 (2009).
Doonan, C. J., Tranchemontagne, D. J., Glover, T. G., Hunt, J. R. & Yaghi, O. M. Exceptional ammonia uptake by a covalent organic framework. Nat. Chem. 2, 235–238 (2010).
Zeng, Y., Zou, R. & Zhao, Y. Covalent organic frameworks for CO2 capture. Adv. Mater. 28, 2855–2873 (2016).
Zhao, Y., Yao, K. X., Teng, B., Zhang, T. & Han, Y. A perfluorinated covalent triazine-based framework for highly selective and water-tolerant CO2 capture. Energy Environ. Sci. 6, 3684–3692 (2013).
Rabbani, M. G. et al. A 2D mesoporous imine-linked covalent organic framework for high pressure gas storage applications. Chem. Eur. J. 19, 3324–3328 (2013).
Gomes, R., Bhanja, P. & Bhaumik, A. A triazine-based covalent organic polymer for efficient CO2 adsorption. Chem. Commun. 51, 10050–10053 (2015).
Gao, Q. et al. Synthesis of microporous nitrogen-rich covalent-organic framework and its application in CO2 capture. Chin. J. Chem. 33, 90–94 (2015).
Zhao, S. et al. Channel-wall functionalization in covalent organic frameworks for the enhancement of CO2 uptake and CO2/N2 selectivity. RSC Adv. 6, 38774–38781 (2016).
Li, Z. et al. An azine-linked covalent organic framework: synthesis, characterization and efficient gas storage. Chem. Eur. J. 21, 12079–12084 (2015).
Gomes, R. & Bhaumik, A. A new triazine functionalized luminescent covalent organic framework for nitroaromatic sensing and CO2 storage. RSC Adv. 6, 28047–28054 (2016).
Dong, B. et al. Immobilization of ionic liquids to covalent organic frameworks for catalyzing the formylation of amines with CO2 and phenylsilane. Chem. Commun. 52, 7082–7085 (2016).
Li, L., Yang, J., Li, J., Chen, Y. & Li, J. Separation of CO2/CH4 and CH4/N2 mixtures by M/DOBDC: a detailed dynamic comparison with MIL-100(Cr) and activated carbon. Micropor. Mesopor. Mater. 198, 236–246 (2014).
Wu, X. et al. Microwave synthesis and characterization of MOF-74 (M = Ni, Mg) for gas separation. Micropor. Mesopor. Mater. 180, 114–122 (2013).
Mason, J. A. et al. Application of a high-throughput analyzer in evaluating solid adsorbents for post-combustion carbon capture via multicomponent adsorption of CO2, N2, and H2O. J. Am. Chem. Soc. 137, 4787–4803 (2015).
Li, L. et al. High gas storage capacities and stepwise adsorption in a UiO type metal–organic framework incorporating Lewis basic bipyridyl sites. Chem. Commun. 50, 2304–2307 (2014).
Nagai, A. et al. Pore surface engineering in covalent organic frameworks. Nat. Commun. 2, 536 (2011). A general and practical strategy for the functionalization of pore walls of various COFs.
Pachfule, P., Kandambeth, S., Diaz Diaz, D. & Banerjee, R. Highly stable covalent organic framework–Au nanoparticles hybrids for enhanced activity for nitrophenol reduction. Chem. Commun. 50, 3169–3172 (2014).
Leng, W., Ge, R., Dong, B., Wang, C. & Gao, Y. Bimetallic docked covalent organic frameworks with high catalytic performance towards tandem reactions. RSC Adv. 6, 37403–37406 (2016).
Leng, W. et al. Sophisticated design of covalent organic frameworks with controllable bimetallic docking for a cascade reaction. Chem. Eur. J. 22, 9087–9091 (2016).
Xu, H. et al. Catalytic covalent organic frameworks via pore surface engineering. Chem. Commun. 50, 1292–1294 (2014).
Díaz, R., Orcajo, M. G., Botas, J. A., Calleja, G. & Palma, J. Co8-MOF-5 as electrode for supercapacitors. Mater. Lett. 68, 126–128 (2012).
Li, S.-L. & Xu, Q. Metal–organic frameworks as platforms for clean energy. Energy Environ. Sci. 6, 1656–1683 (2013).
Lee, D. Y. et al. Unusual energy storage and charge retention in Co-based metal–organic-frameworks. Micropor. Mesopor. Mater. 153, 163–165 (2012).
Gao, Y. et al. Synthesis of nickel oxalate/zeolitic imidazolate framework-67 (NiC2O4/ZIF-67) as a supercapacitor electrode. New J. Chem. 39, 94–97 (2015).
Liao, H., Ding, H., Li, B., Ai, X. & Wang, C. Covalent–organic frameworks: potential host materials for sulfur impregnation in lithium–sulfur batteries. J. Mater. Chem. A 2, 8854–8858 (2014).
Yang, X. et al. Sulfur impregnated in a mesoporous covalent organic framework for high performance lithium–sulfur batteries. RSC Adv. 5, 86137–86143 (2015).
Sun, M.-H. et al. Applications of hierarchically structured porous materials from energy storage and conversion, catalysis, photocatalysis, adsorption, separation, and sensing to biomedicine. Chem. Soc. Rev. 45, 3479–3563 (2016).
Kitamura, T. et al. Electroactive supramolecular self-assembled fibers comprised of doped tetrathiafulvalene-based gelators. J. Am. Chem. Soc. 127, 14769–14775 (2005).
Braga, D. & Horowitz, G. High-performance organic field-effect transistors. Adv. Mater. 21, 1473–1486 (2009).
Pisula, W., Feng, X. & Müllen, K. Charge-carrier transporting graphene-type molecules. Chem. Mater. 23, 554–567 (2011).
Jin, S. et al. Charge dynamics in a donor–acceptor covalent organic framework with periodically ordered bicontinuous heterojunctions. Angew. Chem. Int. Ed. 52, 2017–2021 (2013).
Jin, S. et al. Creation of superheterojunction polymers via direct polycondensation: segregated and bicontinuous donor–acceptor π-columnar arrays in covalent organic frameworks for long-lived charge separation. J. Am. Chem. Soc. 137, 7817–7827 (2015).
Jiménez, Á. J., Calderón, R. M. K., Rodríguez-Morgade, M. S., Guldi, D. M. & Torres, T. Synthesis, characterization and photophysical properties of a melamine-mediated hydrogen-bound phthalocyanine–perylenediimide assembly. Chem. Sci. 4, 1064–1074 (2013).
Calik, M. et al. Extraction of photogenerated electrons and holes from a covalent organic framework integrated heterojunction. J. Am. Chem. Soc. 136, 17802–17807 (2014).
Gole, B., Bar, A. K., Mallick, A., Banerjee, R. & Mukherjee, P. S. An electron rich porous extended framework as a heterogeneous catalyst for Diels–Alder reactions. Chem. Commun. 49, 7439–7441 (2013).
Costentin, C., Robert, M. & Saveant, J.-M. Catalysis of the electrochemical reduction of carbon dioxide. Chem. Soc. Rev. 42, 2423–2436 (2013).
Kumar, B. et al. Photochemical and photoelectrochemical reduction of CO2 . Annu. Rev. Phys. Chem. 63, 541–569 (2012).
DeBlase, C. R. et al. Rapid and efficient redox processes within 2D covalent organic framework thin films. ACS Nano 9, 3178–3183 (2015).
Wu, H. et al. Flexible and binder-free organic cathode for high-performance lithium–ion batteries. Adv. Mater. 26, 3338–3343 (2014).
Meng, Y., Wu, H., Zhang, Y. & Wei, Z. A flexible electrode based on a three-dimensional graphene network-supported polyimide for lithium–ion batteries. J. Mater. Chem. A 2, 10842–10846 (2014).
Shinde, D. B. et al. A mechanochemically synthesized covalent organic framework as a proton-conducting solid electrolyte. J. Mater. Chem. A 4, 2682–2690 (2016).
Guldi, D. M., Gouloumis, A., Vázquez, P. & Torres, T. Charge-transfer states in strongly coupled phthalocyanine fullerene ensembles. Chem. Commun. 2002, 2056–2057 (2002).
Green, M. A., Emery, K., Hishikawa, Y., Warta, W. & Dunlop, E. D. Solar cell efficiency tables (version 47). Prog. Photovolt. Res. Appl. 24, 3–11 (2016).
Schwinghammer, K. et al. Triazine-based carbon nitrides for visible-light-driven hydrogen evolution. Angew. Chem. Int. Ed. 52, 2435–2439 (2013).
Zhang, J. et al. Synthesis of a carbon nitride structure for visible-light catalysis by copolymerization. Angew. Chem. Int. Ed. 49, 441–444 (2010).
Nguyen, N. T. T. et al. Three-dimensional metal-catecholate frameworks and their ultrahigh proton conductivity. J. Am. Chem. Soc. 137, 15394–15397 (2015).
Yoon, M. et al. High and highly anisotropic proton conductivity in organic molecular porous materials. Angew. Chem. Int. Ed. 50, 7870–7873 (2011).
Bureekaew, S. et al. One-dimensional imidazole aggregate in aluminium porous coordination polymers with high proton conductivity. Nat. Mater. 8, 831–836 (2009).
Hurd, J. A. et al. Anhydrous proton conduction at 150 °C in a crystalline metal–organic framework. Nat. Chem. 1, 705–710 (2009).
Wang, P. et al. Synthesis of conjugated covalent organic frameworks/graphene composite for supercapacitor electrodes. RSC Adv. 5, 27290–27294 (2015).
Huang, Y.-B., Pachfule, P., Sun, J.-K. & Xu, Q. From covalent–organic frameworks to hierarchically porous B-doped carbons: a molten-salt approach. J. Mater. Chem. A 4, 4273–4279 (2016).
Yang, H. et al. High conductive two-dimensional covalent organic framework for lithium storage with large capacity. ACS Appl. Mater. Interfaces 8, 5366–5375 (2016).
Bai, L. et al. Nanoscale covalent organic frameworks as smart carriers for drug delivery. Chem. Commun. 52, 4128–4131 (2016).
Mitra, S. et al. Self-exfoliated guanidinium-based ionic covalent organic nanosheets (iCONs). J. Am. Chem. Soc. 138, 2823–2828 (2016).
Smith, B. J. & Dichtel, W. R. Mechanistic studies of two-dimensional covalent organic frameworks rapidly polymerized from initially homogenous conditions. J. Am. Chem. Soc. 136, 8783–8789 (2014).
Smith, B. J., Hwang, N., Chavez, A. D., Novotney, J. L. & Dichtel, W. R. Growth rates and water stability of 2D boronate ester covalent organic frameworks. Chem. Commun. 51, 7532–7535 (2015).
Smith, B. J., Overholts, A. C., Hwang, N. & Dichtel, W. R. Insight into the crystallization of amorphous imine-linked polymer networks to 2D covalent organic frameworks. Chem. Commun. 52, 3690–3693 (2016).
Huang, W. et al. Solvothermal synthesis of microporous, crystalline covalent organic framework nanofibers and their colorimetric nanohybrid structures. ACS Appl. Mater. Interfaces 5, 8845–8849 (2013).
Yi, J. et al. Green, scalable and morphology controlled synthesis of nanofibrous covalent organic frameworks and their nanohybrids through a vapor-assisted solid-state approach. J. Mater. Chem. A 2, 8201–8204 (2014).
Pachfule, P., Kandmabeth, S., Mallick, A. & Banerjee, R. Hollow tubular porous covalent organic framework (COF) nanostructures. Chem. Commun. 51, 11717–11720 (2015).
Kandambeth, S. et al. Self-templated chemically stable hollow spherical covalent organic framework. Nat. Commun. 6, 6786 (2015).
Yang, C.-X., Liu, C., Cao, Y.-M. & Yan, X.-P. Facile room-temperature solution-phase synthesis of a spherical covalent organic framework for high-resolution chromatographic separation. Chem. Commun. 51, 12254–12257 (2015).
Huang, N., Ding, X., Kim, J., Ihee, H. & Jiang, D. A photoresponsive smart covalent organic framework. Angew. Chem. Int. Ed. 54, 8704–8707 (2015).
Dong, W.-l. et al. Substrate orientation effect in the on-surface synthesis of tetrathiafulvalene-integrated single-layer covalent organic frameworks. Langmuir 31, 11755–11759 (2015).
Liu, C., Zhang, W., Zeng, Q. & Lei, S. A photoresponsive surface covalent organic framework: surface-confined synthesis, isomerization, and controlled guest capture and release. Chem. Eur. J. 22, 6768–6773 (2016).
Kissel, P., Murray, D. J., Wulftange, W. J., Catalano, V. J. & King, B. T. A nanoporous two-dimensional polymer by single-crystal-to-single-crystal photopolymerization. Nat. Chem. 6, 774–778 (2014).
Kory, M. J. et al. Gram-scale synthesis of two-dimensional polymer crystals and their structure analysis by X-ray diffraction. Nat. Chem. 6, 779–784 (2014).
Cai, S.-L. et al. The organic flatland — recent advances in synthetic 2D organic layers. Adv. Mater. 27, 5762–5770 (2015).
Kang, Z. et al. Mixed matrix membranes (MMMs) comprising exfoliated 2D covalent organic frameworks (COFs) for efficient CO2 separation. Chem. Mater. 28, 1277–1285 (2016).
Biswal, B. P., Chaudhari, H. D., Banerjee, R. & Kharul, U. K. Chemically stable covalent organic framework (COF)-polybenzimidazole hybrid membranes: enhanced gas separation through pore modulation. Chem. Eur. J. 22, 4695–4699 (2016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Huang, N., Wang, P. & Jiang, D. Covalent organic frameworks: a materials platform for structural and functional designs. Nat Rev Mater 1, 16068 (2016). https://doi.org/10.1038/natrevmats.2016.68
Published:
DOI: https://doi.org/10.1038/natrevmats.2016.68
This article is cited by
-
Multistate structures in a hydrogen-bonded polycatenation non-covalent organic framework with diverse resistive switching behaviors
Nature Communications (2024)
-
Co-immobilization of whole cells and enzymes by covalent organic framework for biocatalysis process intensification
Nature Communications (2024)
-
Reversible modulation of interlayer stacking in 2D copper-organic frameworks for tailoring porosity and photocatalytic activity
Nature Communications (2024)
-
Exploring modern developments in diverse 2D photocatalysts for water oxidation
Journal of Porous Materials (2024)
-
Covalent organic frameworks for CO2 adsorption: fundamentals, structural features and synthesis
Journal of Porous Materials (2024)