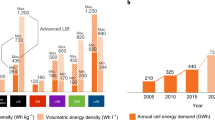
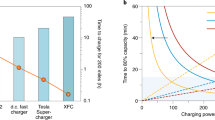
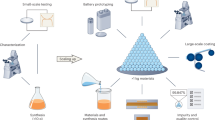
Abstract
Energy density is the main property of rechargeable batteries that has driven the entire technology forward in past decades. Lithium-ion batteries (LIBs) now surpass other, previously competitive battery types (for example, lead–acid and nickel metal hydride) but still require extensive further improvement to, in particular, extend the operation hours of mobile IT devices and the driving mileages of all-electric vehicles. In this Review, we present a critical overview of a wide range of post-LIB materials and systems that could have a pivotal role in meeting such demands. We divide battery systems into two categories: near-term and long-term technologies. To provide a realistic and balanced perspective, we describe the operating principles and remaining issues of each post-LIB technology, and also evaluate these materials under commercial cell configurations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Tarascon, J. M. & Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001).
Etacheri, V., Marom, R., Elazari, R., Salitra, G. & Aurbach, D. Challenges in the development of advanced Li-ion batteries: a review. Energy Environ. Sci. 4, 3243–3262 (2011).
International Energy Agency. Global EV outlook. Understanding the electric vehicle landscape to 2020 (IEA, 2013).
Armand, M. & Tarascon, J. M. Building better batteries. Nature 451, 652–657 (2008).
Bruce, P. G., Freunberger, S. A., Hardwick, L. J. & Tarascon, J.-M. Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 11, 19–29 (2012). The operating principles, advantages and remaining issues of Li–S and Li–O2 batteries are comprehensively reviewed in this article.
Cabana, J., Monconduit, L., Larcher, D. & Palacín, M. R. Beyond intercalation-based Li-ion batteries: the state of the art and challenges of electrode materials reacting through conversion reactions. Adv. Mater. 22, E170–E192 (2010).
Dunn, B., Kamath, H. & Tarascon, J.-M. Electrical energy storage for the grid: a battery of choices. Science 334, 928–935 (2011).
Goodenough, J. B. & Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 22, 587–603 (2010).
Thackeray, M. M., Wolverton, C. & Isaacs, E. D. Electrical energy storage for transportation—approaching the limits of, and going beyond, lithium-ion batteries. Energy Environ. Sci. 5, 7854–7863 (2012).
Duduta, M. et al. Semi-solid lithium rechargeable flow battery. Adv. Energy Mater. 1, 511–516 (2011).
Howard, W. F. & Spotnitz, R. M. Theoretical evaluation of high-energy lithium metal phosphate cathode materials in Li-ion batteries. J. Power Sources 165, 887–891 (2007).
Yazami, R. & Touzain, P. A reversible graphite–lithium negative electrode for electrochemical generators. J. Power Sources 9, 365–371 (1983).
Winter, M., Besenhard, J. O., Spahr, M. E. & Novák, P. Insertion electrode materials for rechargeable lithium batteries. Adv. Mater. 10, 725–763 (1998).
Huggins, R. A. Lithium alloy negative electrodes. J. Power Sources 81–82, 13–19 (1999).
Beaulieu, L. Y., Eberman, K. W., Turner, R. L., Krause, L. J. & Dahn, J. R. Colossal reversible volume changes in lithium alloys. Electrochem. Solid State Lett. 4, A137–A140 (2001).
Wu, H. & Cui, Y. Designing nanostructured Si anodes for high energy lithium ion batteries. Nano Today 7, 414–429 (2012). In this article, issues originating from the volume expansion of Si active materials and the solutions based on nanostructural designs are discussed.
McDowell, M. T., Lee, S. W., Nix, W. D. & Cui, Y. 25th anniversary article: understanding the lithiation of silicon and other alloying anodes for lithium-ion batteries. Adv. Mater. 25, 4966–4985 (2013).
Nelson, P. A. et al. High-performance batteries for off-peak energy storage and electric-vehicle propulsion, progress report. (Argonne National Laboratory, 1976).
Sharma, R. A. & Seefurth, R. N. Thermodynamic properties of the lithium–silicon system. J. Electrochem. Soc. 123, 1763–1768 (1976).
Seefurth, R. N. & Sharma, R. A. Investigation of lithium utilization from a lithium–silicon electrode. J. Electrochem. Soc. 124, 1207–1214 (1977).
Wilson, A. M. & Dahn, J. R. Lithium insertion in carbons containing nanodispersed silicon. J. Electrochem. Soc. 142, 326–332 (1995).
Yu, Y. et al. Reversible storage of lithium in silver-coated three-dimensional macroporous silicon. Adv. Mater. 22, 2247–2250 (2010).
Hwang, T. H., Lee, Y. M., Kong, B.-S., Seo, J.-S. & Choi, J. W. Electrospun core–shell fibers for robust silicon nanoparticle-based lithium ion battery anodes. Nano Lett. 12, 802–807 (2012).
Liu, N. et al. A yolk-shell design for stabilized and scalable Li-ion battery alloy anodes. Nano Lett. 12, 3315–3321 (2012).
Jung, D. S., Hwang, T. H., Park, S. B. & Choi, J. W. Spray drying method for large-scale and high-performance silicon negative electrodes in Li-ion batteries. Nano Lett. 13, 2092–2097 (2013).
Son, I. H. et al. Silicon carbide-free graphene growth on silicon for lithium-ion battery with high volumetric energy density. Nat. Commun. 6, 7393 (2015).
Koo, B. et al. A highly cross-linked polymeric binder for high-performance silicon negative electrodes in lithium ion batteries. Angew. Chem. Int. Ed. Engl. 51, 8762–8767 (2012).
Kwon, T.-w. et al. Systematic molecular-level design of binders incorporating meldrum's acid for silicon anodes in lithium rechargeable batteries. Adv. Mater. 26, 7979–7985 (2014).
Wang, C. et al. Self-healing chemistry enables the stable operation of silicon microparticle anodes for high-energy lithium-ion batteries. Nat. Chem. 5, 1042–1048 (2013).
Chen, Z. et al. High-areal-capacity silicon electrodes with low-cost silicon particles based on spatial control of self-healing binder. Adv. Energy Mater. 5, 1401826 (2015).
Li, J., Lewis, R. B. & Dahn, J. R. Sodium carboxymethyl cellulose: a potential binder for Si negative electrodes for Li-ion batteries. Electrochem. Solid State Lett. 10, A17–A20 (2007).
Kovalenko, I. et al. A major constituent of brown algae for use in high-capacity Li-ion batteries. Science 334, 75–79 (2011).
Murase, M. et al. Crop-derived polysaccharides as binders for high-capacity silicon/graphite-based electrodes in lithium-ion batteries. ChemSusChem 5, 2307–2311 (2012).
Jeong, Y. K. et al. Hyperbranched β-cyclodextrin polymer as an effective multidimensional binder for silicon anodes in lithium rechargeable batteries. Nano Lett. 14, 864–870 (2014).
Jeong, Y. K. et al. Millipede-inspired structural design principle for high performance polysaccharide binders in silicon anodes. Energy Environ. Sci. 8, 1224–1230 (2015).
Liu, G. et al. Polymers with tailored electronic structure for high capacity lithium battery electrodes. Adv. Mater. 23, 4679–4683 (2011).
Wu, H. et al. Stable Li-ion battery anodes by in situ polymerization of conducting hydrogel to conformally coat silicon nanoparticles. Nat. Commun. 4, 1943 (2013).
Erickson, E. M. et al. Review — development of advanced rechargeable batteries: a continuous challenge in the choice of suitable electrolyte solutions. J. Electrochem. Soc. 162, A2424–A2438 (2015).
Etacheri, V. et al. Exceptional electrochemical performance of Si nanowires in 1,3-dioxolane solutions: a surface chemical investigation. Langmuir 28, 6175–6184 (2012).
Markevich, E. et al. Amorphous columnar silicon anodes for advanced high voltage lithium ion full cells: dominant factors governing cycling performance. J. Electrochem. Soc. 160, A1824–A1833 (2013).
Markevich, E. et al. High performance of thick amorphous columnar monolithic film silicon anodes in ionic liquid electrolytes at elevated temperature. RSC Adv. 4, 48572–48575 (2014).
Fukuoka, H., Aramata, M. & Miyawaki, S. Method for producing SiOx (x < 1). US Patent 0254102 (2007).
DeWet Erasmus, H. & Persson, J. A. Preparation and properties of silicon monoxide. J. Electrochem. Soc. 95, 316–318 (1949).
Park, E. et al. Dual-size silicon nanocrystal-embedded SiOx nanocomposite as a high-capacity lithium storage material. ACS Nano 9, 7690–7696 (2015).
Doh, C.-H. et al. A new SiO/C anode composition for lithium-ion battery. J. Power Sources 179, 367–370 (2008).
Zhao, J. et al. Dry-air-stable lithium silicide–lithium oxide core–shell nanoparticles as high-capacity prelithiation reagents. Nat. Commun. 5, 5088 (2014).
Liu, N., Hu, L., McDowell, M. T., Jackson, A. & Cui, Y. Prelithiated silicon nanowires as an anode for lithium ion batteries. ACS Nano 5, 6487–6493 (2011).
Kim, H. J. et al. Controlled prelithiation of silicon monoxide for high performance lithium-ion rechargeable full cells. Nano Lett. 16, 282–288 (2015).
Miyachi, M., Yamamoto, H., Kawai, H., Ohta, T. & Shirakata, M. Analysis of SiO anodes for lithium-ion batteries. J. Electrochem. Soc. 152, A2089–A2091 (2005).
Komaba, S. et al. Study on polymer binders for high-capacity SiO negative electrode of Li-ion batteries. J. Phys. Chem. C 115, 13487–13495 (2011).
Rossen, E., Jones, C. D. W. & Dahn, J. R. Structure and electrochemistry of LixMnyNi1−yO2 . Solid State Ionics 57, 311–318 (1992).
Rossouw, M. H., Liles, D. C. & Thackeray, M. M. Synthesis and structural characterization of a novel layered lithium manganese oxide, Li0.36Mn0.91O2, and its lithiated derivative, Li1.09Mn0.91O2 . J. Solid State Chem. 104, 464–466 (1993).
Chikkannanavar, S. B., Bernardi, D. M. & Liu, L. A review of blended cathode materials for use in Li-ion batteries. J. Power Sources 248, 91–100 (2014).
Jung, S.-K. et al. Understanding the degradation mechanisms of LiNi0.5Co0.2Mn0.3O2 cathode material in lithium ion batteries. Adv. Energy Mater. 4, 1300787 (2014).
Chen, C. H. et al. Aluminum-doped lithium nickel cobalt oxide electrodes for high-power lithium-ion batteries. J. Power Sources 128, 278–285 (2004).
Manthiram, A., Knight, J. C., Myung, S.-T., Oh, S.-M. & Sun, Y.-K. Nickel-rich and lithium-rich layered oxide cathodes: progress and perspectives. Adv. Energy Mater. 6, 1501010 (2015). This article addresses key challenges in the synthesis and battery operation of high-capacity layered oxide cathodes and outlines future research directions.
Liu, W. et al. Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries. Angew. Chem. Int. Ed. Engl. 54, 4440–4457 (2015).
Lin, F. et al. Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries. Nat. Commun. 5, 3529 (2014).
Johnson, C. S., Li, N., Lefief, C., Vaughey, J. T. & Thackeray, M. M. Synthesis, characterization and electrochemistry of lithium battery electrodes: xLi2MnO3·(1−x)LiMn0.333Ni0.333Co0.333O2 (0 ≤ x ≤ 0.7). Chem. Mater. 20, 6095–6106 (2008).
Lee, K.-S., Myung, S.-T., Amine, K., Yashiro, H. & Sun, Y.-K. Structural and electrochemical properties of layered Li[Ni1−2 x CoxMnx]O2 (x = 0.1–0.3) positive electrode materials for Li-ion batteries. J. Electrochem. Soc. 154, A971–A977 (2007).
Nayak, P. K., Grinblat, J., Levi, M., Markovsky, B. & Aurbach, D. Structural and electrochemical evidence of layered to spinel phase transformation of Li and Mn rich layered cathode materials of the formulae xLi[Li1/3Mn2/3]O2·(1−x)LiMn1/3Ni1/3Co1/3O2 (x = 0.2, 0.4, 0.6) upon cycling. J. Electrochem. Soc. 161, A1534–A1547 (2014).
Haik, O. et al. On the surface chemistry of LiMO2 cathode materials (M = [MnNi] and [MnNiCo]): electrochemical, spectroscopic, and calorimetric studies. J. Electrochem. Soc. 157, A1099–A1107 (2010).
Kam, K. C., Mehta, A., Heron, J. T. & Doeff, M. M. Electrochemical and physical properties of Ti-substituted layered nickel manganese cobalt oxide (NMC) cathode materials. J. Electrochem. Soc. 159, A1383–A1392 (2012).
Karan, N. et al. Structural characteristics and electrochemical performance of layered Li[Mn0.5−xCr2xNi0.5−x]O2 cathode materials. J. Power Sources 187, 586–590 (2009).
Kim, J. & Amine, K. A comparative study on the substitution of divalent, trivalent and tetravalent metal ions in LiNi1− xMxO2 (M = Cu2+, Al3+ and Ti4+). J. Power Sources 104, 33–39 (2002).
Kim, H.-B. et al. Electrochemical and thermal characterization of AlF3-coated Li[Ni0.8Co0.15Al0.05]O2 cathode in lithium-ion cells. J. Power Sources 179, 347–350 (2008).
West, W. et al. Electrochemical behavior of layered solid solution Li2MnO3−LiMO2 (M = Ni, Mn, Co) Li-ion cathodes with and without alumina coatings. J. Electrochem. Soc. 158, A883–A889 (2011).
Zhang, X. et al. Structural and electrochemical study of Al2O3 and TiO2 coated Li1.2Ni0.13Mn0.54Co0.13O2 cathode material using ALD. Adv. Energy Mater. 3, 1299–1307 (2013).
Cho, J., Kim, H. & Park, B. Comparison of overcharge behavior of AlPO4-coated LiCoO2 and LiNi0.8Co0.1Mn0.1O2 cathode materials in Li-ion cells. J. Electrochem. Soc. 151, A1707–A1711 (2004).
Ma, X., Wang, C., Han, X. & Sun, J. Effect of AlPO4 coating on the electrochemical properties of LiNi0.8Co0.2O2 cathode material. J. Alloys Compd. 453, 352–355 (2008).
Liu, J., Wang, Q., Reeja-Jayan, B. & Manthiram, A. Carbon-coated high capacity layered Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathodes. Electrochem. Commun. 12, 750–753 (2010).
Chen, Y., Zhang, Y., Chen, B., Wang, Z. & Lu, C. An approach to application for LiNi0.6Co0.2Mn0.2O2 cathode material at high cutoff voltage by TiO2 coating. J. Power Sources 256, 20–27 (2014).
Zheng, J., Li, J., Zhang, Z., Guo, X. & Yang, Y. The effects of TiO2 coating on the electrochemical performance of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material for lithium-ion battery. Solid State Ionics 179, 1794–1799 (2008).
Cho, Y., Lee, S., Lee, Y., Hong, T. & Cho, J. Spinel-layered core–shell cathode materials for Li-ion batteries. Adv. Energy Mater. 1, 821–828 (2011).
Wu, F. et al. Ultrathin spinel membrane-encapsulated layered lithium-rich cathode material for advanced Li-ion batteries. Nano Lett. 14, 3550–3555 (2014).
Sun, Y.-K. et al. Nanostructured high-energy cathode materials for advanced lithium batteries. Nat. Mater. 11, 942–947 (2012).
Whittingham, M. S. Electrical energy storage and intercalation chemistry. Science 192, 1126–1127 (1976).
Aurbach, D., Zinigrad, E., Cohen, Y. & Teller, H. A short review of failure mechanisms of lithium metal and lithiated graphite anodes in liquid electrolyte solutions. Solid State Ionics 148, 405–416 (2002).
Xu, W. et al. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 7, 513–537 (2014). The factors that affect undesirable dendrite growth and poor Coulombic efficiency in Li-metal anodes are summarized in this article, along with recent developments to mitigate the problem.
Aurbach, D., Zinigrad, E., Teller, H. & Dan, P. Factors which limit the cycle life of rechargeable lithium (metal) batteries. J. Electrochem. Soc. 147, 1274–1279 (2000).
Yu, X., Bates, J. B., Jellison, G. E. & Hart, F. X. A stable thin-film lithium electrolyte: lithium phosphorus oxynitride. J. Electrochem. Soc. 144, 524–532 (1997).
Kozen, A. C. et al. Next-generation lithium metal anode engineering via atomic layer deposition. ACS Nano 9, 5884–5892 (2015).
Lee, H., Lee, D. J., Kim, Y.-J., Park, J.-K. & Kim, H.-T. A simple composite protective layer coating that enhances the cycling stability of lithium metal batteries. J. Power Sources 284, 103–108 (2015).
Zheng, G. et al. Interconnected hollow carbon nanospheres for stable lithium metal anodes. Nat. Nanotechnol. 9, 618–623 (2014).
Kim, J.-S., Kim, D. W., Jung, H. T. & Choi, J. W. Controlled lithium dendrite growth by a synergistic effect of multilayered graphene coating and an electrolyte additive. Chem. Mater. 27, 2780–2787 (2015).
Ryou, M.-H. et al. Excellent cycle life of lithium-metal anodes in lithium-ion batteries with mussel-inspired polydopamine-coated separators. Adv. Energy Mater. 2, 645–650 (2012).
Lu, Y., Tu, Z. & Archer, L. A. Stable lithium electrodeposition in liquid and nanoporous solid electrolytes. Nat. Mater. 13, 961–969 (2014).
Aurbach, D. et al. Design of electrolyte solutions for Li and Li-ion batteries: a review. Electrochim. Acta 50, 247–254 (2004).
Ota, H., Shima, K., Ue, M. & Yamaki, J.-i. Effect of vinylene carbonate as additive to electrolyte for lithium metal anode. Electrochim. Acta 49, 565–572 (2004).
Lee, Y. M. et al. Effects of triacetoxyvinylsilane as SEI layer additive on electrochemical performance of lithium metal secondary battery. Electrochem. Solid State Lett. 10, A216–A219 (2007).
Ding, F. et al. Dendrite-free lithium deposition via self-healing electrostatic shield mechanism. J. Am. Chem. Soc. 135, 4450–4456 (2013).
Ryou, M.-H., Lee, Y. M., Lee, Y., Winter, M. & Bieker, P. Mechanical surface modification of lithium metal: towards improved Li metal anode performance by directed Li plating. Adv. Funct. Mater. 25, 834–841 (2015).
Lee, J. H. et al. Effect of lithium powder size on the performance of lithium-powder/lithium trivanadate secondary batteries shown via impedance analysis. Electrochim. Acta 131, 202–206 (2014).
Rao, M. L. B. Organic electrolyte cells. US Patent 3413154 (1968).
Rauh, R. D., Abraham, K. M., Pearson, G. F., Surprenant, J. K. & Brummer, S. B. Lithium-dissolved sulfur battery with an organic electrolyte. J. Electrochem. Soc. 126, 523–527 (1979).
Aurbach, D. et al. On the surface chemical aspects of very high energy density, rechargeable Li–sulfur batteries. J. Electrochem. Soc. 156, A694–A702 (2009).
Elazari, R. et al. Morphological and structural studies of composite sulfur electrodes upon cycling by HRTEM, AFM and Raman spectroscopy. J. Electrochem. Soc. 157, A1131–A1138 (2010).
Ji, X. & Nazar, L. F. Advances in Li–S batteries. J. Mater. Chem. 20, 9821–9826 (2010).
Yin, Y.-X., Xin, S., Guo, Y.-G. & Wan, L.-J. Lithium–sulfur batteries: electrochemistry, materials, and prospects. Angew. Chem. Int. Ed. Engl. 52, 13186–13200 (2013).
Ji, X., Lee, K. T. & Nazar, L. F. A highly ordered nanostructured carbon–sulphur cathode for lithium–sulphur batteries. Nat. Mater. 8, 500–506 (2009).
Elazari, R., Salitra, G., Garsuch, A., Panchenko, A. & Aurbach, D. Sulfur-impregnated activated carbon fiber cloth as a binder-free cathode for rechargeable Li–S batteries. Adv. Mater. 23, 5641–5644 (2011).
Evers, S., Yim, T. & Nazar, L. F. Understanding the nature of absorption/adsorption in nanoporous polysulfide sorbents for the Li–S battery. J. Phys. Chem. C 116, 19653–19658 (2012).
Song, J. et al. Nitrogen-doped mesoporous carbon promoted chemical adsorption of sulfur and fabrication of high-areal-capacity sulfur cathode with exceptional cycling stability for lithium–sulfur batteries. Adv. Funct. Mater. 24, 1243–1250 (2014).
Xin, S. et al. Smaller sulfur molecules promise better lithium–sulfur batteries. J. Am. Chem. Soc. 134, 18510–18513 (2012).
Kim, J.-S., Hwang, T. H., Kim, B. G., Min, J. & Choi, J. W. A lithium–sulfur battery with a high areal energy density. Adv. Funct. Mater. 24, 5359–5367 (2014).
Chung, W. J. et al. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat. Chem. 5, 518–524 (2013).
Nagao, M., Hayashi, A. & Tatsumisago, M. Electrochemical performance of all-solid-state Li/S batteries with sulfur-based composite electrodes prepared by mechanical milling at high temperature. Energy Technol. 1, 186–192 (2013).
Machida, N., Kobayashi, K., Nishikawa, Y. & Shigematsu, T. Electrochemical properties of sulfur as cathode materials in a solid-state lithium battery with inorganic solid electrolytes. Solid State Ionics 175, 247–250 (2004).
Kinoshita, S., Okuda, K., Machida, N., Naito, M. & Sigematsu, T. All-solid-state lithium battery with sulfur/carbon composites as positive electrode materials. Solid State Ionics 256, 97–102 (2014).
Kobayashi, T. et al. All solid-state battery with sulfur electrode and thio-LISICON electrolyte. J. Power Sources 182, 621–625 (2008).
Unemoto, A. et al. Development of bulk-type all-solid-state lithium–sulfur battery using LiBH4 electrolyte. Appl. Phys. Lett. 105, 083901 (2014).
Marmorstein, D. et al. Electrochemical performance of lithium/sulfur cells with three different polymer electrolytes. J. Power Sources 89, 219–226 (2000).
Ryu, H.-S., Ahn, H.-J., Kim, K.-W., Ahn, J.-H. & Lee, J.-Y. Discharge process of Li/PVdF/S cells at room temperature. J. Power Sources 153, 360–364 (2006).
Choi, J. W. et al. Microporous poly(vinylidene fluoride-co-hexafluoropropylene) polymer electrolytes for lithium/sulfur cells. J. Ind. Eng. Chem. 12, 939–949 (2006).
Rao, M., Geng, X., Li, X., Hu, S. & Li, W. Lithium–sulfur cell with combining carbon nanofibers–sulfur cathode and gel polymer electrolyte. J. Power Sources 212, 179–185 (2012).
Koh, J. Y. et al. Electrochemical reduction mechanism of sulfur particles electrically isolated from carbon cathodes of lithium–sulfur cells. J. Electrochem. Soc. 161, A2117–A2120 (2014).
Markevich, E. et al. The effect of a solid electrolyte interphase on the mechanism of operation of lithium–sulfur batteries. J. Mater. Chem. A 3, 19873–19883 (2015).
Markevich, E. et al. Fluoroethylene carbonate as an important component in organic carbonate electrolyte solutions for lithium sulfur batteries. Electrochem. Commun. 60, 42–46 (2015).
Yuan, Z. et al. Hierarchical free-standing carbon-nanotube paper electrodes with ultrahigh sulfur-loading for lithium–sulfur batteries. Adv. Funct. Mater. 24, 6105–6112 (2014).
Abraham, K. M. & Jiang, Z. A polymer electrolyte-based rechargeable lithium/oxygen battery. J. Electrochem. Soc. 143, 1–5 (1996).
Li, Y. & Dai, H. Recent advances in zinc–air batteries. Chem. Soc. Rev. 43, 5257–5275 (2014). Detailed effects of cell components in primary and secondary Zn–air batteries on the electrochemical performance are discussed in this article, focusing on the cells' operation principles, technical issues and potential solutions.
Palmer, N. J. Secondary metal/air cell. US Patent 3650837 (1972).
Shao, Y. et al. Making Li–air batteries rechargeable: material challenges. Adv. Funct. Mater. 23, 987–1004 (2013).
Hasegawa, S. et al. Study on lithium/air secondary batteries — stability of NASICON-type lithium ion conducting glass–ceramics with water. J. Power Sources 189, 371–377 (2009).
Visco, S. et al. Aqueous and nonaqueous lithium–air batteries enabled by water-stable lithium metal electrodes. J. Solid State Electrochem. 18, 1443–1456 (2014).
Zhang, T. et al. Li/polymer electrolyte/water stable lithium-conducting glass ceramics composite for lithium–air secondary batteries with an aqueous electrolyte. J. Electrochem. Soc. 155, A965–A969 (2008).
Visco, S. J., Katz, B. D., Nimon, Y. S. & De Jonghe, L. C. Protected active metal electrode and battery cell structures with non-aqueous interlayer architecture. US Patent 7282295 (2007).
Kim, B. G. et al. Improved reversibility in lithium–oxygen battery: understanding elementary reactions and surface charge engineering of metal alloy catalyst. Sci. Rep. 4, 4225 (2014).
Freunberger, S. A. et al. The lithium–oxygen battery with ether-based electrolytes. Angew. Chem. Int. Ed. Engl. 50, 8609–8613 (2011).
Ottakam Thotiyl, M. M., Freunberger, S. A., Peng, Z. & Bruce, P. G. The carbon electrode in nonaqueous Li–O2 cells. J. Am. Chem. Soc. 135, 494–500 (2013).
Kim, B. G., Kim, S., Lee, H. & Choi, J. W. Wisdom from the human eye: a synthetic melanin radical scavenger for improved cycle life of Li–O2 battery. Chem. Mater. 26, 4757–4764 (2014).
Peng, Z., Freunberger, S. A., Chen, Y. & Bruce, P. G. A reversible and higher-rate Li–O2 battery. Science 337, 563–566 (2012).
Harding, J. R., Lu, Y.-C., Tsukada, Y. & Shao-Horn, Y. Evidence of catalyzed oxidation of Li2O2 for rechargeable Li–air battery applications. Phys. Chem. Chem. Phys. 14, 10540–10546 (2012).
Li, F. et al. Ru/ITO: a carbon-free cathode for nonaqueous Li–O2 battery. Nano Lett. 13, 4702–4707 (2013).
Lu, J. et al. A nanostructured cathode architecture for low charge overpotential in lithium–oxygen batteries. Nat. Commun. 4, 2383 (2013).
Débart, A., Paterson, A. J., Bao, J. & Bruce, P. G. α-MnO2 nanowires: a catalyst for the O2 electrode in rechargeable lithium batteries. Angew. Chem. Int. Ed. Engl. 47, 4521–4524 (2008).
Black, R., Lee, J.-H., Adams, B., Mims, C. A. & Nazar, L. F. The role of catalysts and peroxide oxidation in lithium–oxygen batteries. Angew. Chem. Int. Ed. Engl. 52, 392–396 (2013).
Shang, C. et al. Compatible interface design of CoO-based Li–O2 battery cathodes with long-cycling stability. Sci. Rep. 5, 8335 (2015).
McCloskey, B. D. et al. On the efficacy of electrocatalysis in nonaqueous Li–O2 batteries. J. Am. Chem. Soc. 133, 18038–18041 (2011).
Grande, L. et al. The lithium/air battery: still an emerging system or a practical reality? Adv. Mater. 27, 784–800 (2015).
Adams, B. D. et al. Current density dependence of peroxide formation in the Li–O2 battery and its effect on charge. Energy Environ. Sci. 6, 1772–1778 (2013).
Chen, Y., Freunberger, S. A., Peng, Z., Fontaine, O. & Bruce, P. G. Charging a Li–O2 battery using a redox mediator. Nat. Chem. 5, 489–494 (2013).
Lim, H.-D. et al. Superior rechargeability and efficiency of lithium–oxygen batteries: hierarchical air electrode architecture combined with a soluble catalyst. Angew. Chem. Int. Ed. Engl. 53, 3926–3931 (2014).
Freunberger, S. A. et al. Reactions in the rechargeable lithium–O2 battery with alkyl carbonate electrolytes. J. Am. Chem. Soc. 133, 8040–8047 (2011).
Xu, D. Wang, Z.-l., Xu, J.-j., Zhang, L.-l. & Zhang, X.-b. Novel DMSO-based electrolyte for high performance rechargeable Li–O2 batteries. Chem. Commun. 48, 6948–6950 (2012).
Sharon, D. et al. On the challenge of electrolyte solutions for Li–air batteries: monitoring oxygen reduction and related reactions in polyether solutions by spectroscopy and EQCM. J. Phys. Chem. Lett. 4, 127–131 (2013).
Sharon, D. et al. Oxidation of dimethyl sulfoxide solutions by electrochemical reduction of oxygen. J. Phys. Chem. Lett. 4, 3115–3119 (2013).
Sharon, D. et al. Lithium–oxygen electrochemistry in non-aqueous solutions. Isr. J. Chem. 55, 508–520 (2015).
Kwak, W.-J. et al. Understanding the behavior of Li–oxygen cells containing LiI. J. Mater. Chem. A 3, 8855–8864 (2015).
Ottakam Thotiyl, M. M. et al. A stable cathode for the aprotic Li–O2 battery. Nat. Mater. 12, 1050–1056 (2013).
Kundu, D., Black, R., Berg, E. J. & Nazar, L. F. A highly active nanostructured metallic oxide cathode for aprotic Li–O2 batteries. Energy Environ. Sci. 8, 1292–1298 (2015).
Su, D., Dou, S. & Wang, G. Single crystalline Co3O4 nanocrystals exposed with different crystal planes for Li–O2 batteries. Sci. Rep. 4, 5767 (2014).
Kwak, W.-J. et al. A Mo2C/carbon nanotube composite cathode for lithium–oxygen batteries with high energy efficiency and long cycle life. ACS Nano 9, 4129–4137 (2015).
Li, F. et al. Superior performance of a Li–O2 battery with metallic RuO2 hollow spheres as the carbon-free cathode. Adv. Energy Mater. 5, 1500294 (2015).
Kim, B. G., Lee, J.-N., Lee, D. J., Park, J.-K. & Choi, J. W. Robust cycling of Li–O2 batteries through the synergistic effect of blended electrolytes. ChemSusChem 6, 443–448 (2013).
Linden, D. & Reddy, T. B. Handbook of Batteries (McGraw-Hill, 2001).
Zhang, J., Zhao, Z., Xia, Z. & Dai, L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat. Nanotechnol. 10, 444–452 (2015).
Parker, J. F., Chervin, C. N., Nelson, E. S., Rolison, D. R. & Long, J. W. Wiring zinc in three dimensions re-writes battery performance-dendrite-free cycling. Energy Environ. Sci. 7, 1117–1124 (2014).
Müller, S., Haas, O., Schlatter, C. & Comninellis, C. Development of a 100 W rechargeable bipolar zinc/oxygen battery. J. Appl. Electrochem. 28, 305–310 (1998).
Vorkapic´, L. Ž., Dražic´, D. M. & Despic´, A. R. Corrosion of pure and amalgamated zinc in concentrated alkali hydroxide solutions. J. Electrochem. Soc. 121, 1385–1392 (1974).
Lee, C. W., Sathiyanarayanan, K., Eom, S. W. & Yun, M. S. Novel alloys to improve the electrochemical behavior of zinc anodes for zinc/air battery. J. Power Sources 160, 1436–1441 (2006).
Ein-Eli, Y., Auinat, M. & Starosvetsky, D. Electrochemical and surface studies of zinc in alkaline solutions containing organic corrosion inhibitors. J. Power Sources 114, 330–337 (2003).
Cho, Y.-D. & Fey, G. T.-K. Surface treatment of zinc anodes to improve discharge capacity and suppress hydrogen gas evolution. J. Power Sources 184, 610–616 (2008).
Li, Y. et al. Advanced zinc–air batteries based on high-performance hybrid electrocatalysts. Nat. Commun. 4, 1805 (2013).
Cheng, H.-H. & Tan, C.-S. Reduction of CO2 concentration in a zinc/air battery by absorption in a rotating packed bed. J. Power Sources 162, 1431–1436 (2006).
Yabuuchi, N., Kubota, K., Dahbi, M. & Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 114, 11636–11682 (2014). This article details the motivation behind Na-ion batteries and provides a summary of the broad range of their positive and negative electrodes.
Kim, Y., Ha, K. H., Oh, S. M. & Lee, K. T. High-capacity anode materials for sodium-ion batteries. Chem. Eur. J. 20, 11980–11992 (2014).
Delmas, C., Braconnier, J.-J., Fouassier, C. & Hagenmuller, P. Electrochemical intercalation of sodium in NaxCoO2 bronzes. Solid State Ionics 3–4, 165–169 (1981).
Yabuuchi, N. et al. P2-type NaxFe1/2Mn1/2O2 made from earth-abundant elements for rechargeable Na batteries. Nat. Mater. 11, 512–517 (2012).
Ong, S. P. et al. Voltage, stability and diffusion barrier differences between sodium-ion and lithium-ion intercalation materials. Energy Environ. Sci. 4, 3680–3688 (2011).
Yu, C. Y. et al. NaCrO2 cathode for high-rate sodium-ion batteries. Energy Environ. Sci. 8, 2019–2026 (2015).
de la Llave, E. et al. Comparison between Na-ion and Li-ion cells: understanding the critical role of the cathodes stability and the anodes pretreatment on the cells behavior. ACS Appl. Mater. Interfaces 8, 1867–1875 (2015).
Delmas, C., Cherkaoui, F., Nadiri, A. & Hagenmuller, P. A. NASICON-type phase as intercalation electrode: NaTi2(PO4)3 . Mater. Res. Bull. 22, 631–639 (1987).
Senguttuvan, P., Rousse, G., Seznec, V., Tarascon, J.-M. & Rosa Palacin, M. Na2Ti3O7: lowest voltage ever reported oxide insertion electrode for sodium ion batteries. Chem. Mater. 23, 4109–4111 (2011).
Rudola, A., Saravanan, K., Devaraj, S., Gong, H. & Balaya, P. Na2Ti6O13: a potential anode for grid-storage sodium-ion batteries. Chem. Commun. 49, 7451–7453 (2013).
Wu, D. et al. NaTiO2: a layered anode material for sodium-ion batteries. Energy Environ. Sci. 8, 195–202 (2015).
Chevrier, V. L. & Ceder, G. Challenges for Na-ion negative electrodes. J. Electrochem. Soc. 158, A1011–A1014 (2011).
Baggetto, L. et al. Characterization of sodium ion electrochemical reaction with tin anodes: experiment and theory. J. Power Sources 234, 48–59 (2013).
Ellis, L. D., Hatchard, T. D. & Obrovac, M. N. Reversible insertion of sodium in tin. J. Electrochem. Soc. 159, A1801–A1805 (2012).
Kim, Y. et al. An amorphous red phosphorus/carbon composite as a promising anode material for sodium ion batteries. Adv. Mater. 25, 3045–3049 (2013).
Qian, J., Wu, X., Cao, Y., Ai, X. & Yang, H. High capacity and rate capability of amorphous phosphorus for sodium ion batteries. Angew. Chem. Int. Ed. Engl. 52, 4633–4636 (2013).
Li, W.-J., Chou, S.-L., Wang, J.-Z., Liu, H.-K. & Dou, S.-X. Simply mixed commercial red phosphorus and carbon nanotube composite with exceptionally reversible sodium-ion storage. Nano Lett. 13, 5480–5484 (2013).
Darwiche, A. et al. Better cycling performances of bulk Sb in Na-ion batteries compared to Li-ion systems: an unexpected electrochemical mechanism. J. Am. Chem. Soc. 135, 10179–10179 (2013).
Baggetto, L. et al. Intrinsic thermodynamic and kinetic properties of Sb electrodes for Li-ion and Na-ion batteries: experiment and theory. J. Mater. Chem. A 1, 7985–7994 (2013).
He, M., Kraychyk, K., Walter, M. & Kovalenko, M. V. Monodisperse antimony nanocrystals for high-rate Li-ion and Na-ion battery anodes: nano versus bulk. Nano Lett. 14, 1255–1262 (2014).
Baggetto, L., Keum, J. K., Browning, J. F. & Veith, G. M. Germanium as negative electrode material for sodium-ion batteries. Electrochem. Commun. 34, 41–44 (2013).
Abel, P. R. et al. Nanocolumnar germanium thin films as a high-rate sodium-ion battery anode material. J. Phys. Chem. C 117, 18885–18890 (2013).
Webb, S. A., Baggetto, L., Bridges, C. A. & Veith, G. M. The electrochemical reactions of pure indium with Li and Na: anomalous electrolyte decomposition, benefits of FEC additive, phase transitions and electrode performance. J. Power Sources 248, 1105–1117 (2014).
Baggetto, L., Marszewski, M., Gorka, J., Jaroniec, M. & Veith, G. M. AlSb thin films as negative electrodes for Li-ion and Na-ion batteries. J. Power Sources 243, 699–705 (2013).
Baggetto, L., Allcorn, E., Manthiram, A. & Veith, G. M. Cu2Sb thin films as anode for Na-ion batteries. Electrochem. Commun. 27, 168–171 (2013).
Baggetto, L., Allcorn, E., Unocic, R. R., Manthiram, A. & Veith, G. M. Mo3Sb7 as a very fast anode material for lithium-ion and sodium-ion batteries. J. Mater. Chem. A 1, 11163–11169 (2013).
Koo, B. et al. Intercalation of sodium ions into hollow iron oxide nanoparticles. Chem. Mater. 25, 245–252 (2013).
Alcántara, R., Jaraba, M., Lavela, P. & Tirado, J. L. NiCo2O4 spinel: first report on a transition metal oxide for the negative electrode of sodium-ion batteries. Chem. Mater. 14, 2847–2848 (2002).
Su, D., Wang, C., Ahn, H. & Wang, G. Octahedral tin dioxide nanocrystals as high capacity anode materials for Na-ion batteries. Phys. Chem. Chem. Phys. 15, 12543–12550 (2013).
Yu, D. Y. W. et al. High-capacity antimony sulphide nanoparticle-decorated graphene composite as anode for sodium-ion batteries. Nat. Commun. 4, 2922 (2013).
Zhu, C., Mu, X., van Aken, P. A., Yu, Y. & Maier, J. Single-layered ultrasmall nanoplates of MoS2 embedded in carbon nanofibers with excellent electrochemical performance for lithium and sodium storage. Angew. Chem. Int. Ed. Engl. 53, 2152–2156 (2014).
Fullenwarth, J., Darwiche, A., Soares, A., Donnadieu, B. & Monconduit, L. NiP3: a promising negative electrode for Li- and Na-ion batteries. J. Mater. Chem. A 2, 2050–2059 (2014).
Kim, Y. et al. Tin phosphide as a promising anode material for Na-ion batteries. Adv. Mater. 26, 4139–4144 (2014).
Hong, S. Y. et al. Charge carriers in rechargeable batteries: Na ions versus Li ions. Energy Environ. Sci. 6, 2067–2081 (2013).
Deng, W. et al. A low cost, all-organic Na-ion battery based on polymeric cathode and anode. Sci. Rep. 3, 2671 (2013).
Park, Y. et al. Sodium terephthalate as an organic anode material for sodium ion batteries. Adv. Mater. 24, 3562–3567 (2012).
Hwang, J.-Y. et al. Radially aligned hierarchical columnar structure as a cathode material for high energy density sodium-ion batteries. Nat. Commun. 6, 6865 (2015).
Aurbach, D. et al. Progress in rechargeable magnesium battery technology. Adv. Mater. 19, 4260–4267 (2007).
Yoo, H. D. et al. Mg rechargeable batteries: an on-going challenge. Energy Environ. Sci. 6, 2265–2279 (2013). Progress in rechargeable Mg batteries since the original prototype work are reviewed in this article, which focuses on the development of electrolyte solutions and cathode materials.
Gregory, T. D., Hoffman, R. J. & Winterton, R. C. Nonaqueous electrochemistry of magnesium: applications to energy storage. J. Electrochem. Soc. 137, 775–780 (1990).
Aurbach, D. et al. Prototype systems for rechargeable magnesium batteries. Nature 407, 724–727 (2000).
Pour, N., Gofer, Y., Major, D. T. & Aurbach, D. Structural analysis of electrolyte solutions for rechargeable Mg batteries by stereoscopic means and DFT calculations. J. Am. Chem. Soc. 133, 6270–6278 (2011).
Doe, R. E. et al. Novel, electrolyte solutions comprising fully inorganic salts with high anodic stability for rechargeable magnesium batteries. Chem. Commun. 50, 243–245 (2014).
Lv, D. et al. A scientific study of current collectors for Mg batteries in Mg(AlCl2EtBu)2/THF electrolyte. J. Electrochem. Soc. 160, A351–A355 (2013).
Zhang, R. et al. α-MnO2 as a cathode material for rechargeable Mg batteries. Electrochem. Commun. 23, 110–113 (2012).
Imamura, D., Miyayama, M., Hibino, M. & Kudo, T. Mg intercalation properties into V2O5 gel/carbon composites under high-rate condition. J. Electrochem. Soc. 150, A753–A758 (2003).
Nam, K. W. et al. The high performance of crystal water containing manganese birnessite cathodes for magnesium batteries. Nano Lett. 15, 4071–4079 (2015).
Liang, Y. et al. Rechargeable Mg batteries with graphene-like MoS2 cathode and ultrasmall Mg nanoparticle anode. Adv. Mater. 23, 640–643 (2011).
Liu, B. et al. Rechargeable Mg-ion batteries based on WSe2 nanowire cathodes. ACS Nano 7, 8051–8058 (2013).
Orikasa, Y. et al. High energy density rechargeable magnesium battery using earth-abundant and non-toxic elements. Sci. Rep. 4, 5622 (2014).
Lukatskaya, M. R. et al. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 341, 1502–1505 (2013).
Levi, M. D. et al. Solving the capacitive paradox of 2D MXene using electrochemical quartz-crystal admittance and in situ electronic conductance measurements. Adv. Energy Mater. 5, 1400815 (2015).
Wang, R. Y., Wessells, C. D., Huggins, R. A. & Cui, Y. Highly reversible open framework nanoscale electrodes for divalent ion batteries. Nano Lett. 13, 5748–5752 (2013).
Mizuno, Y. et al. Electrochemical Mg2+ intercalation into a bimetallic CuFe Prussian blue analog in aqueous electrolytes. J. Mater. Chem. A 1, 13055–13059 (2013).
Gaddum, L. W. & French, H. E. The electrolysis of Grignard solutions. J. Am. Chem. Soc. 49, 1295–1299 (1927).
Kim, H. S. et al. Structure and compatibility of a magnesium electrolyte with a sulphur cathode. Nat. Commun. 2, 427 (2011).
Guo, Y.-s. et al. Boron-based electrolyte solutions with wide electrochemical windows for rechargeable magnesium batteries. Energy Environ. Sci. 5, 9100–9106 (2012).
Shterenberg, I. et al. Evaluation of (CF3SO2)2N− (TFSI) based electrolyte solutions for Mg batteries. J. Electrochem. Soc. 162, A7118–A7128 (2015).
Mohtadi, R., Matsui, M., Arthur, T. S. & Hwang, S.-J. Magnesium borohydride: from hydrogen storage to magnesium battery. Angew. Chem. Int. Ed. Engl. 51, 9780–9783 (2012).
Carter, T. J. et al. Boron clusters as highly stable magnesium-battery electrolytes. Angew. Chem. Int. Ed. Engl. 53, 3173–3177 (2014).
Tutusaus, O. et al. An efficient halogen-free electrolyte for use in rechargeable magnesium batteries. Angew. Chem. Int. Ed. Engl. 54, 7900–7904(2015).
Acknowledgements
J.W.C. thanks J. Min for his help in the volumetric-energy-density evaluation. J.W.C. acknowledges the support of the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (NRF-2012-R1A2A1A01011970 and NRF-2014R1A4A1003712). D.A. acknowledges help from the Israel Science Foundation, in the framework of the INREP project. This work was also made possible by NPRP grant #5-569-2–232 from the Qatar National Research Fund (a member of the Qatar Foundation).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Choi, J., Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat Rev Mater 1, 16013 (2016). https://doi.org/10.1038/natrevmats.2016.13
Published:
DOI: https://doi.org/10.1038/natrevmats.2016.13
This article is cited by
-
High voltage electrolytes for lithium-ion batteries with micro-sized silicon anodes
Nature Communications (2024)
-
The Promise of 3D Printed Solid Polymer Electrolytes for Developing Sustainable Batteries: A Techno-Commercial Perspective
International Journal of Precision Engineering and Manufacturing-Green Technology (2024)
-
Post lithium-sulfur battery era: challenges and opportunities towards practical application
Science China Chemistry (2024)
-
Progress and perspective on rechargeable magnesium-ion batteries
Science China Chemistry (2024)
-
Mn-based cathode materials for rechargeable batteries
Science China Chemistry (2024)