Abstract
Neurotrophic factors, particularly brain-derived neurotrophic factor (BDNF) and other members of the neurotrophin family, are central mediators of the activity-dependent plasticity through which environmental experiences, such as sensory information are translated into the structure and function of neuronal networks. Synthesis, release and action of BDNF is regulated by neuronal activity and BDNF in turn leads to trophic effects such as formation, stabilization and potentiation of synapses through its high-affinity TrkB receptors. Several clinically available drugs activate neurotrophin signaling and neuronal plasticity. In particular, antidepressant drugs rapidly activate TrkB signaling and gradually increase BDNF expression, and the behavioral effects of antidepressants are mediated by and dependent on BDNF signaling through TrkB at least in rodents. These findings indicate that antidepressants, widely used drugs, effectively act as TrkB activators. They further imply that neuronal plasticity is a central mechanism in the action of antidepressant drugs. Indeed, it was recently discovered that antidepressants reactivate a state of plasticity in the adult cerebral cortex that closely resembles the enhanced plasticity normally observed during postnatal critical periods. This state of induced plasticity, known as iPlasticity, allows environmental stimuli to beneficially reorganize networks abnormally wired during early life. iPlasticity has been observed in cortical as well as subcortical networks and is induced by several pharmacological and non-pharmacological treatments. iPlasticity is a new pharmacological principle where drug treatment and rehabilitation cooperate; the drug acts permissively to enhance plasticity and rehabilitation provides activity to guide the appropriate wiring of the plastic network. Optimization of iPlastic drug treatment with novel means of rehabilitation may help improve the efficacy of available drug treatments and expand the use of currently existing drugs into new indications.
Similar content being viewed by others
Introduction
Neuronal plasticity is a process through which external and internal environment of an individual gradually becomes represented in neuronal structure and function during development and through learning. Although gross connectivity develops through genetically governed guidance, fine-tuning takes place through experience and activity-dependent plasticity, where neurons and connections that actively participate in network function are selected for stabilization and strengthened, whereas inactive contacts are weakened or eliminated.1, 2, 3
Neuronal plasticity does not only involve trophic processes such as neurogenesis and synaptogenesis but also includes atrophic processes, such as the elimination of inactive neurons and neuronal contacts. Although it is often thought that loss of neurons or synapses is harmful, elimination of connections that do not mediate useful information is, in fact, necessary for the optimal signal-to-noise ratio within the nervous system.1, 2 Indeed, most of the neurons and synapses formed during development are wiped out by adulthood.1, 2 Therefore, plasticity in itself does not have any particular direction; it is the experience-dependent activity within the neuronal network that determines which of the connections are strengthened and maintained and which ones are eliminated. Therefore, plasticity is adaptive when it is guided by beneficial environmental stimuli, but it can also be maladaptive, if the guiding experiences are adverse.
Neuronal plasticity is heightened during critical periods of postnatal development, which allows an efficient experience-driven fine-tuning of developing networks.4 After the closure of critical periods, neuronal plasticity and changes in network structure are more restricted. However, recent data indicate that several drugs used for the treatment of neuropsychiatric disorders can directly influence the plasticity and reactivate a critical period-like plasticity in the adult brain, a process known as induced plasticity (iPlasticity).5, 6, 7, 8
To be translated into neuronal structure and function, neuronal activity needs molecular mediators9 and neurotrophic factors are prime candidates for mediators between neuronal activity and plasticity.10, 11 In this review, we will first introduce the role of the neurotrophin family and especially of BDNF as a mediator of plasticity and drug effects. We will then discuss the role of neuronal plasticity in the mechanisms of action of drugs acting on the brain. Finally, we will review recent evidence that developmental-like plasticity, iPlasticity, can be activated in the adult brain and argue that iPlastic drugs should be combined with training, rehabilitation or psychotherapy to facilitate treatment outcome. For the role of other neurotrophic factors in neuronal plasticity, especially the family members of the glial cell line-derived neurotrophic factor, fibroblast growth factor and insulin-like growth factor, we refer to recent review articles.12, 13, 14
Neurotrophins in plasticity
The first neurotrophic factors, nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) were discovered through their ability to support the survival of neurons and neurites during development;15, 16, 17 other members of the neurotrophin family, neurotrophin-3 (NT-3)18, 19, 20 and neurotrophin-4 (NT-4)21, 22, 23 were then identified through sequence similarity to NGF and BDNF.
Neurotrophins act by binding to two types of receptors, Trk-family members24, 25, 26, 27 and the p75 neurotrophin receptor (p75NTR).28, 29, 30, 31 NGF binds to TrkA, BDNF and NT-4 to TrkB and NT-3 interacts mainly with TrkC receptors, whereas all neurotrophin family members bind to the p75NTR.32 Recent evidence suggests that mature forms of neurotrophins predominantly bind to Trk receptors to promote neuronal survival and plasticity, whereas intracellularly generated pro-forms of neurotrophins preferentially interact with the p75NTR to increase cell death and eliminate synapses.33, 34
In the peripheral nervous system, neurotrophic factors are constitutively released in minute amounts by the target cells to regulate survival and process outgrowth of developing neurons. To act as a mediator of activity-dependent plasticity in the central nervous system, neurotrophins need to be released and to act in an activity-dependent manner.10, 35, 36 Levi-Montalcini and co-workers first found that hypothalamic Ngf mRNA levels in mice were regulated by behavioral activity, namely aggression, indicating the role for neurotrophins in the regulation of behavior.37 At the same time, Gall and co-workers showed that Ngf mRNA was regulated by neuronal activity induced by seizures.38 A similar activity-dependent regulation was observed for Bdnf mRNA,39, 40, 41 and subsequent studies showed that synthesis and release of BDNF and NGF, but not those of NT-3 and NT-4, are regulated by activity.9 Furthermore, TrkB, which is mostly localized within vesicles inside the cell, is translocated to plasma membrane through neuronal activity.42, 43 These data are consistent with a central role of neurotrophins as mediators of activity-dependent plasticity9, 10 (Figure 1).
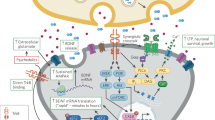
Neurotrophins as regulators of activity-dependent plasticity. Neurotrophins and their pro-forms mediate synaptic strengthening and dendritic retraction, respectively. This dual action is regulated by neuronal activity that controls the expression and secretion of BDNF, cleavage of proBDNF and plasma membrane translocation of TrkB receptors. BDNF binds to TrkB receptors to mediate neuronal survival and stabilization, whereas proBDNF binds with a higher affinity to p75NTRs that in the absence of Trk receptors promote apoptosis, dendritic retraction and synaptic depression, allowing differential responses in neurons depending on their activity state. When two axons are competing for the innervation of the same target neuron, the functional connection eventually forms between the two active neurons and the less active neuron retracts. Neuronal activity promotes the release of BDNF and proBDNF as well as tPA, which promotes extracellular cleavage of proBDNF to BDNF. In addition, TrkB receptors that normally reside inside the cell are inserted to the plasma membrane in the active neurons. This allows BDNF to mediate survival promoting and synapse strengthening signals in the active neurons. In the less active neuron, TrkB receptors or tPA are not available; however, p75NTR is expressed and the proBDNF released from the active neighbor or the target cell promotes atrophic signals. These effects result in activity-dependent selection of neuronal connectivity. BDNF, brain-derived neurotrophic factor; ES, endoplasmic system; p75, p75 neurotrophin receptor; proBDNF, pro-form of BDNF; tPA, tissue plasminogen activator; TrkB, tropomyosin receptor kinase.
The first functional evidence for the role of neurotrophins in plasticity was obtained in the visual cortex, a classical model for developmental plasticity, when Maffei and colleagues showed that NGF prevents the effects of monocular deprivation (MD) during the critical period of visual development44, 45 (for a review, see refs 46, 47). These findings led the authors to propose an influential hypothesis that thalamic axons reaching the visual cortex compete for access to a neurotrophic factor that is regulated by activity.44 The observation that BDNF synthesis in the visual cortex is regulated by visual stimulation made BDNF the prime candidate for this activity-dependent regulated factor.48 Indeed, the presence of an excess of the TrkB ligands, BDNF and NT-4, prevented ocular column segregation during development,49, 50, 51, 52 which is consistent with such a role. These studies demonstrate that neurotrophins are critical regulators of neuronal plasticity in the developing visual cortex;53 they also established visual cortex as an excellent model for studying the role of neurotrophic factors in neuronal plasticity (Figure 2).
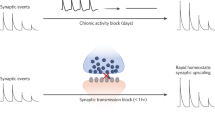
iPlasticity in the adult visual cortex. Development of the ocular dominance (OD) and its response to monocular deprivation in the mammalian visual cortex is probably the best-characterized model of neuronal network development in the cerebral cortex. (a) During early life, thalamic inputs representing each eye diffusely innervate the entire visual cortex, but (b) during the CP of early postnatal life, visual inputs from each eye segregate into alternating eye-specific regions in the primary visual cortex, called OD columns, such that each column becomes predominant innervated by one eye only. (c) If vision of one eye is blocked (MD) during the critical period, the more active inputs of the open eye take over the visual cortex through an activity-dependent competition involving BDNF signaling, and the closed eye loses its connectivity (see Figure 1), thereby becoming poor in vision, or amblyopic. Vision of the amblyopic eye can be recovered if normal vision is restored during the critical period and the use of the weaker eye is encouraged by patching the better eye, but if MD extends beyond the end of the critical period, amblyopia becomes permanent and cannot be revised by patching. (d) However, vision of an amblyopic eye can be restored in adulthood if eye patching is combined with a treatment that induces iPlasticity, such as fluoxetine or environmental enrichment (see Table 1). iPlasticity promotes functional recovery also in other brain areas where abnormal environment has lead to miswiring of developing networks. Modified with permission from Elsevier Ltd from Castrén and Hen.3 BDNF, brain-derived neurotrophic factor; CP, critical period; MD, monocular deprivation.
NGF and BDNF promote the outgrowth of axons and dendrites and increase synapse formation.54, 55, 56, 57 The effect of BDNF on dendritic branching is dependent on neuronal activity,56 which again is consistent with the role of BDNF in activity-dependent plasticity.
BDNF also regulates synaptic plasticity. Long-term potentiation (LTP) is a widely used model of synaptic plasticity and BDNF has been demonstrated to be a critical regulator of the late, protein synthesis-dependent stage of LTP.9, 58, 59 High-frequency stimulation that induces LTP increases BDNF production.60, 61 Furthermore, BDNF increases neurotransmitter release,62 promotes synaptic transmission and LTP in vitro63, 64, 65 as well as in vivo.66, 67, 68, 69 Mice deficient of BDNF fail to show LTP,70, 71 which can be rescued by exogenous BDNF administration.71, 72, 73 Similar findings have been found in mice carrying the Val66Met mutation in the Bdnf gene,74 which corresponds to a common human BDNF gene polymorphism that alters the amino acid valine to methionine in the position 66 of the BDNF pro-region, leading to impaired activity-dependent BDNF release.75 Consistent with this, direct current stimulation promotes learning in homozygous BDNF Val66Val subjects, but not in Met allele carriers, both in humans and mice.76 However, since long-term manipulations, such as transgenic mice, can have major effects on glutamate release and other basic synaptic properties that in turn alter the threshold for LTP and other forms of synaptic plasticity, it is not always clear whether the effects of neurotrophins on neuronal or synaptic plasticity are instructive or permissive.59, 77
Mice deficient in TrkB also show blunted LTP and deficient learning.58, 78 These effects appear to be specifically mediated by signaling through the phospholipase Cγ signaling pathway, since deletion of the tyrosine residue in TrkB mediating this signaling disrupts LTP.79 Furthermore, overexpression of TrkB occludes LTP in the hippocampal CA1 area, which is consistent with a critical role for TrkB signaling in LTP.80
As noted above, plasticity is not only a trophic effect but also includes atrophic processes such as apoptosis, axonal retraction, dendritic pruning, synapse loss and long-term depression. Interestingly, genes for neurotrophins produce mediators of both of these functions. Although mature forms of neurotrophins activate Trk family receptors to promote trophic effects, pro-forms of neurotrophins bind to and activate the p75NTR to induce atrophic effects.31, 33, 81 p75NTR is widely expressed in cortical and hippocampal neurons during early development, but its expression is reduced during the first postnatal weeks; however, there is evidence that p75NTR is re-expressed in the cortex and hippocampus after injury.31, 82, 83 In addition, the BDNF pro-peptide that is released from pro-BDNF has recently been shown to possess biological effects on its own.84, 85 Pro-neurotrophins induce apoptosis in the absence of Trk receptors via interaction between sortilin and p75NTR.33, 82, 86, 87 Pro-BDNF binding to p75NTR has also been shown to induce pruning of axons and dendrites85, 88, 89, 90, 91 and promote long-term depression.92, 93, 94 There is evidence that the release of these competing signals derived from a single pro-neurotrophin is effectively utilized by neurons competing for target innervation to promote their own growth while inhibiting that of competing neighbors81, 95, 96, 97, 98 (Figure 1).
Neurotrophins and plasticity in drug responses
Since the discovery of BDNF and its receptor TrkB, efforts have been made to use BDNF as a therapeutic agent for neuropsychiatric disorders or to develop small-molecule pharmaceuticals that could increase the production of BDNF or activate TrkB in brain (for review, see refs 99, 100), and these efforts are still ongoing.101 It is, therefore, remarkable that there actually are safe and widely used drugs already in market that increase the production of BDNF or the activation of TrkB in brain. In particular, the effects of antidepressant drugs on BDNF synthesis and TrkB signaling have been well-characterized;102, 103, 104, 105 yet these effects have been largely ignored by the pharmaceutical industry and clinical development. Given the meager outcome of the efforts to develop BDNF mimetics or TrkB agonists, one cannot escape contemplating that many of the scientists involved in these efforts ended up being treated with the very drugs they failed to discover.
Antidepressants activate BDNF signaling
Duman and co-workers were the first to observe that antidepressant drugs acting through diverse mechanisms increased Bdnf mRNA expression in the rat hippocampus.106 Later, multiple studies reported that chronic antidepressant treatment increases Bdnf mRNA expression107, 108, 109 and triggers upregulation of genes associated with BDNF-induced plasticity.110, 111, 112 The antidepressant-induced increase in Bdnf mRNA expression can be further potentiated if the drug treatment is combined with voluntary exercise.113 Moreover, chronic antidepressant treatment can prevent the downregulation of Bdnf mRNA expression caused by stress.106, 109, 113 In addition, BDNF protein levels as well as the expression of genes associated with BDNF-induced plasticity are increased after chronic antidepressant treatment.112, 114 Acute antidepressant treatment, however, does not regulate BDNF expression.106, 114, 115, 116, 117 Studies on human subjects support the findings in rodents; BDNF expression is increased in post-mortem samples of the hippocampus of depressed patients on antidepressant medication at the time of death.118 Furthermore, electroconvulsive shock treatment increases brain and serum BDNF levels in rats and depressed patients, respectively.106, 119 Conversely, BDNF and TrkB mRNA and protein levels are decreased in the frontal cortex and hippocampus of suicide victims when compared with controls.120
Genetic studies have demonstrated that BDNF signaling is required for the behavioral effects of antidepressant drugs.105 In BDNF heterozygous knockout mice and BDNF conditional knockout mice, the behavioral response to antidepressant drugs in the forced swim test is abolished.105, 115, 121 Moreover, after chronic mild stress, BDNF heterozygous knockout mice did not respond to chronic imipramine treatment in the novelty suppressed feeding test.122 On the other hand, administration of BDNF directly into the midbrain region123 or hippocampus is sufficient to induce antidepressant-like behavior and neurogenesis.69, 124
The increase in BDNF expression takes several days of antidepressant treatment to develop, but a single acute administration of many different antidepressants rapidly increases the phosphorylation of TrkB.115, 116, 117 This delayed increase in BDNF expression may be mediated by TrkB activation, since BDNF is known to regulate its own expression through TrkB.125, 126 Antidepressants increase TrkB phosphorylation at the phospholipase Cγ-binding site (Y816), but not at the Shc-binding tyrosine (Y515) and the phosphorylation takes place independent of BDNF,117 indicating TrkB transactivation. Interestingly, fluoxetine activates TrkB receptor similarly in wild-type mice and mice lacking the serotonin transporter, the main target of fluoxetine.117 TrkB receptor overexpression, which results in increased TrkB signaling, is sufficient to produce antidepressant-like behavioral effect in the forced swim test.127 Intact TrkB signaling is required for the behavioral effects of antidepressant drugs, since mice overexpressing the dominant-negative truncated form of the TrkB receptor do not respond to antidepressant drugs in the forced swim test.115 Moreover, the behavioral effects of chronic antidepressant treatment were blunted in mice lacking TrkB in the newly born hippocampal neurons.128 In addition to activating TrkB receptor signaling, chronic treatment with antidepressant drugs increases Trkb mRNA expression.106
Chronic stress is an important precipitating factor for depression and has been shown to induce atrophic changes in dendritic complexity and thus affect neuronal network function. Interestingly in animal models, antidepressant treatment during the chronic stress paradigm can counteract the effects of stress on dendritic spines via a mechanism that includes regulation of glucocorticoid receptor phosphorylation by BDNF.129 Specifically, BDNF through TrkB activation regulates the functional consequences of the glucocorticoid receptor activation following stress.130
Taken together, these data convincingly demonstrate that antidepressant drugs activate TrkB signaling and subsequently increase BDNF levels in brain, and that BDNF signaling is critical for the behavioral effects of antidepressants. Therefore, effective TrkB agonists exist and are therapeutically used by millions of patients.
Antidepressants and neuronal plasticity
The finding that BDNF expression is increased by antidepressants was the trigger for the hypothesis that neuronal plasticity is involved in the antidepressant drug action.102 Since then, several lines of data indicate that antidepressant drugs activate and act through neuronal plasticity.3 Neurogenesis in adult animals is restricted to the subventricular zone of lateral ventricles and to the dentate gyrus of the hippocampus.131 Hippocampal neurogenesis is sensitive to a variety of environmental stimuli, including exercise, enrichment and antidepressant treatment.131 Essentially all antidepressant drugs increase hippocampal neurogenesis after ~2 weeks of treatment in rodents132, 133, 134, 135 and neurogenesis appears to be required for many, although not all the behavioral effects of antidepressant drugs.133, 136, 137 The effects of antidepressants on neurogenesis are dependent on the intact BDNF signaling through TrkB.128, 138
In addition to hippocampal neurogenesis, antidepressants increase remodeling of axons and dendrites in the hippocampus110, 139 as well as in the prefrontal cortex.137 Antidepressants also increase synaptogenesis apparently by increasing spine turnover.137, 140, 141 Furthermore, antidepressants promote LTP in the hippocampus, cortex and amygdala5, 139, 142, 143 and propagation of signals through hippocampal circuitry.144, 145 Although the effects of antidepressants on dendritic remodeling in the dentate gyrus are associated with ongoing neurogenesis,139 in other parts of the hippocampus and in the prefrontal cortex these effects are independent of neurogenesis.137
Ketamine
Ketamine has recently received a lot of attention as a rapid-acting antidepressant drug146 (for a review, see ref. 147). A single dose of ketamine alleviates depression within an hour, but the effect lasts for a week or more, even though the half-life of ketamine and its metabolites is only a few hours.146, 147 This temporal discrepancy between the effects and kinetics of ketamine suggests that neuronal plasticity may be behind the long-lasting effects.148 Indeed, ketamine promotes synaptogenesis in the rodent prefrontal cortex.148, 149 Ketamine has also been reported to promote hippocampal neurogenesis,150, 151 but neurogenesis does not appear to be necessary for the antidepressant effects of this drug.151
BDNF and TrkB have been linked to the mechanism of the antidepressant actions of ketamine.103, 148 BDNF Val66Met polymorphism can affect the responsiveness to ketamine in humans and rodents. The ability of ketamine to induce dendritic spine formation was abolished in BDNF Met66Met mice and the mice failed to respond to ketamine in FST.152 Depressed human patients carrying the met allele did not respond to ketamine as effectively as the Val66Val carriers.153 These results suggest that the activity-dependent secretion, which is impaired in BDNF Met carriers, is involved in the mechanism of ketamine action. In cultured neurons, ketamine stimulation indeed increases BDNF secretion.154
The ketamine metabolite, (2R,6R)-hydroxynorketamine (HNK), can reproduce the behavioral effects of ketamine in animal models,155 although it does not bind to NMDA receptors that are thought to be the main targets mediating the effects of ketamine. Instead, (2R,6R)-HNK increases AMPA-mediated currents and BDNF expression. The NMDA receptor-independent actions of (2R,6R)-HNK suggest that mechanisms other than NMDA antagonism, such as the effects mediated by BDNF and TrkB, can be crucial in the antidepressant effects of ketamine. This hypothesis is supported by findings from Autry et al.156 that a single ketamine injection rapidly increases the TrkB receptor activation and the translation of BDNF via eukaryotic elongation factor 2 in the mouse hippocampus, effects that are also seen after chronic fluoxetine treatment.157 Furthermore, the antidepressant-like behavioral effects of ketamine were lost in mice lacking BDNF or TrkB or if BDNF function blocking antibody was infused into the medial prefrontal cortex of rats.154, 156 In BDNF heterozygous knockout mice, however, the response to ketamine in FST is preserved, suggesting that ketamine is able to produce antidepressant effects in situations where BDNF expression is reduced but not completely lost.158
Fingolimod
Fingolimod is a sphingosine-1-phosphate receptor modulator that is clinically used for multiple sclerosis, but it has been suggested for the treatment of several neurological disorders, including Huntington’s disease and Rett syndrome, where BDNF signaling has been implicated.159 Indeed, fingolimod increases BDNF levels in cultured neurons and in brain and rescues reduced BDNF levels in the brain of Rett syndrome model mice.160 In addition, fingolimod has been shown to improve memory and prevent the upregulation of p75NTR in Huntington’s disease model mice.161 Finally, fingolimod has antidepressant-like effects,162 which is consistent with the critical role of BDNF signaling in the antidepressant drug action.
Drug-induced Trk receptor transactivation
TrkB receptors can be activated independently of BDNF via transactivation through other receptors. TrkB receptor appears to mediate, in particular, the neuronal survival promoting effects of compounds able to transactivate TrkB.163 For example, the adenosine A2 and PACAP receptors that belong to the family of G-protein-coupled receptors can transactivate Trk receptors.164, 165 In vivo, the effects of adenosine 2 A receptor agonists on survival of motoneurons were mediated via TrkB receptor transactivation.166 In addition to transactivation via G-protein-coupled receptors, TrkB receptor activation and signaling can be induced by glucocorticoids, for example dexamethasone, to mediate their effects on neuronal survival.167
Drugs of abuse
Drugs of abuse produce long-lasting memories, typically persisting for life and neuronal plasticity has been for a long time implicated in the formation and maintenance of these memories (for a thorough discussion of this area, see refs 168, 169, 170). The first evidence for long-term plastic changes in response to an addictive drug was observed in the dopaminergic neurons originating from the ventral tegmental area and forming synapses in the nucleus accumbens (NAc). A single cocaine administration induced an LTP in this synapse that was observed 24 h after the cocaine administration but no longer at 1 week after.171 Repeated cocaine injections also induce production of new synapses in the excitatory projections to NAc, which remain silent expressing only NMDA receptors and having no AMPA-receptor mediated currents.172 Subsequently, during withdrawal, AMPA-receptors are gradually recruited to these silent synapses, which converts them to mature synaptic contacts.173
The role of neurotrophins in addiction has been extensively studied, particularly by Nestler and co-workers.174 BDNF is expressed in dopamine neurons as well as in excitatory neurons projecting to the NAc and TrkB is expressed in both dopamine D1 and D2 receptor expressing neurons in the NAc.174, 175 Expression of both BDNF and TrkB is increased by cocaine exposure176, 177 and inhibition of BDNF-TrkB signaling reduces the rewarding effects of cocaine.177 Interestingly, TrkB signaling within the D2-expressing NAc neurons promotes reward to cocaine, whereas TrkB in the D1 neurons suppresses it.175 In contrast, chronic morphine administration reduces BDNF expression in the ventral tegmental area and suppression of BDNF signaling in the NAc promotes morphine reward.178 Therefore, BDNF as well as other neurotrophic factors (for a review of the role of glial cell line-derived neurotrophic factor, see ref. 179) have a critical, though complex, role in the neuronal plasticity leading to addiction.
It has been proposed that addictive drugs of abuse 'hijack' a process of neuronal plasticity that normally takes place during development. Nestler and co-workers have proposed a neural rejuvenation hypothesis of addiction, which suggests that repeated exposure to cocaine reactivates developmental processes that normally occur in the juvenile brain.170 It will be important to explore whether rejuvenation and iPlasticity in fact represent a common mechanism through which developmental plasticity is activated in the adult brain through drugs or experiences to promote adaptive or maladaptive plasticity.
Role of iPlasticity in drug responses
Plasticity is adaptation of the nervous system to environmental experiences, and since exposure to a drug is an experience, it is to be expected that drugs acting on the nervous system involve plasticity. However, recent evidence suggests that central processes of developmental plasticity, such as critical periods, neurogenesis and synaptic plasticity, can be direct targets of drug treatments.
Postnatal critical or sensitive periods are phases of heightened neuronal plasticity during which a certain network is particularly sensitive to environmental input and experiences have a large impact on the subsequent structure and function of the network.4 A classical experimental set-up for the investigation of critical periods is the development of the mammalian visual cortex4, 180 (Figure 2). In this process a perfectly healthy eye that has grown its projections to the visual cortex normally, may lose its connectivity and become permanently deficient only because it is deprived of activation during the critical period. Critical periods are ubiquitous and also govern the development of, for example, motor learning, language and social interactions.181, 182, 183 Therefore, abnormal experiences during early life can guide maladaptive wiring of many cortical networks, including those governing social interactions, and this wiring becomes permanent after the closure of the corresponding critical period, impeding normal adaptation of the network function. Critical periods typically have a relatively well-defined boundaries and it is known that GABA-mediated signaling as well as BDNF are central regulators of the opening and closure of these boundaries.4, 184, 185 Drugs acting on GABA receptors, such as benzodiazepines and anesthetics, are occasionally used in children and adolescents, but so far very little is known about the impact of these drug treatments on the opening and closure of critical periods, a field that clearly would benefit from more attention.
iPlasticity: induced critical period-like plasticity in the adult brain
It had been considered that once closed, critical periods remain closed. However, recent evidence has demonstrated that it is possible to reactivate a state of plasticity in the adult cerebral cortex closely resembling that observed at the peak of postnatal critical periods.3, 7, 104, 186 This iPlasticity creates a window of opportunity for better rewiring of abnormally connected or injured neuronal networks, which has obvious potential in the treatment of neuronal trauma and psychiatric disorders (Figure 2). Furthermore, since natural critical periods coincide with a period of brain growth when many experimental procedures such as brain imaging are complicated, iPlasticity that can be induced in the adult brain facilitates investigations of the molecular and cellular mechanisms of enhanced plasticity.
The first chemical treatment that was shown to induce critical period-like plasticity in the adult brain was the enzyme chondroitinase ABC (ChABC) that, upon intracerebral infusion, degrades perineuronal nets, extracellular matrix structures preferentially encasing parvalbumin-expressing interneurons.187 Intracerebral infusion of ChABC was shown to reactivate a critical period-like ocular dominance (OD) plasticity after MD in the rat visual cortex.188 Degradation of perineuronal nets has been shown to reactivate early life-like plasticity also in the spinal cord and fear circuitry.189, 190 Subsequently, several pharmacological treatments (Table 1) and environmental manipulations,7, 199, 200, 201 as well as genetic means, have been used to induce iPlasticity in several model systems and also in humans.186 Clearly, iPlasticity is an increasingly recognized pharmacological principle with a wide range of potential applications.
iPlasticity induced by antidepressant drugs
Intracortical infusion of ChABC is an invasive procedure and not feasible for human use. However, recent findings have shown that the antidepressant fluoxetine activates iPlasticity in the adult rat visual cortex in a manner very similar to that found at the peak of the natural critical period and to that produced by ChABC or enriched environment.5, 104, 141 During chronic treatment by fluoxetine, critical period-like plasticity is reactivated and MD induces a shift in OD in the visual cortex of adult rats5 (Figure 2). Further, in rats rendered amblyopic through MD from early life onwards, opening of the weak eye and patching of the healthy eye in adulthood restores vision to the amblyopic eye only when fluoxetine is given during the patching procedure.5 BDNF levels are increased by fluoxetine in the visual cortex and BDNF signaling through TrkB is required for iPlasticity. Furthermore, fluoxetine reduces intracortical inhibition and diazepam that potentiates GABA-mediated inhibition, prevents its effects on visual plasticity.5 Finally, serotonin through 5HT1A receptors is involved in iPlasticity.193 Fluoxetine has also been shown to produce enhanced structural plasticity in the mouse visual cortex, promoting turnover of synaptic sites.141 Essentially all antidepressant drugs increase TrkB signaling in brain,116 but it is not clear whether iPlasticity is produced by other antidepressants than fluoxetine. However, preliminary data show that tianeptine, an antidepressant that does not inhibit monoamine reuptake,202 readily reactivates OD plasticity in the adult rat visual cortex (JF Maya-Vetencourt, A Cattaneo and E Castrén, unpublished), which suggests that iPlasticity might be induced by other antidepressant drugs as well. It should be emphasized that fluoxetine treatment alone has no effects on the vision in rats; the effects on visual acuity only become apparent when the promoted plasticity is combined with a manipulation (such as MD) that produces a new pattern of activity within the plastic networks.5
iPlasticity produced by fluoxetine is not restricted to the visual cortex. Chronic peroral fluoxetine exposure induces markers of critical period-like plasticity and promotes LTP in the amygdala, which is indicative of iPlasticity.143, 203 These effects also reactivate the ability to suppress fearful memories when fluoxetine treatment is combined with extinction training.143 Similar iPlastic effects have also been shown after injection of ChABC into the amygdala in adult mice.190 As was the case for iPlasticity in the visual cortex, the effects of fluoxetine on fear extinction were also dependent on BDNF signaling and were apparent only when fluoxetine treatment was combined with rehabilitation, in this case extinction training.143 Taken together, iPlasticity may constitute a neurobiological basis for the observation that the combination of antidepressant treatment and psychotherapy works better than either treatment alone.143
In the adult hippocampal dentate gyrus, fluoxetine reverts the molecular and functional properties of granule neurons to an immature state, a phenomenon coined dematuration.6, 204 Although dematuration coincides with iPlasticity in other cortical regions, it is currently unclear whether these two phenomena share the same molecular and cellular background.
Fluoxetine has recently been found to promote recovery from stroke and from brain trauma.205, 206, 207 It is possible that iPlasticity has a significant role in these actions. In rats, iPlasticity induced either by ChABC or fluoxetine improves recovery from spinal cord lesion.189, 191 If iPlasticity was the underlying mechanism, fluoxetine (promoting plasticity) and rehabilitation (guiding plasticity by activity) together should produce an even better response than either treatment alone; this should be taken into consideration in patient care and in the design of future clinical trials.
Taken together, these data demonstrate that a safe and widely used drug fluoxetine can reactivate critical period-like plasticity in many cortical regions and promote recovery of vision in an amblyopic eye in adult rodents, but whether similar effects are produced in humans is not clear. Normann and co-workers showed that a 3-week treatment with the antidepressant sertraline increases visually evoked potentials in response to a strong visual stimulation in healthy humans,208 which is reminiscent of the increased LTP produced by fluoxetine in rats,5 indicating that antidepressants influence human visual cortex. However, in a recent clinical trial in amblyopic patients, fluoxetine and placebo were equally effective in improving the acuity of the amblyopic eye when given together with a daily eye patching and a computer game-based training of the weaker eye (H Huttunen, M Palva, L Lindberg, S Palva, V Saarela, J Liinamaa, E Karvonen, M-L Latvala, S Booms, E Castrén and H Uusitalo, unpublished observations). Therefore, it remains unclear to what extent iPlasticity contributes to the clinical effects of antidepressant drugs in humans.
iPlasticity induced by other drug treatments
Experiences have long-lasting effects on gene expression through the epigenetic regulation of chromatin structure and DNA methylation.209, 210 Histone acetylation is one of the key epigenetic mechanisms promoting the open chromatin state and regulating gene expression. Histone acetylation can be increased by inhibitors of histone deacetylase (HDAC) and several drugs in clinical use act as HDAC inhibitors.209 Since activity-dependent plasticity requires changes in gene expression and protein synthesis, HDAC inhibitors might activate iPlasticity. Indeed, HDAC inhibition increases the expression of plasticity-related factors and promotes OD plasticity in the visual cortex.193, 194, 195 Treatment with the HDAC inhibitor valproate also reactivates the plasticity related to music preference in mice through activation of plasticity in a network including the prefrontal cortex.196 Interestingly, valproate promotes auditory pitch recognition in human volunteers;197 since development of perfect pitch has a postnatal critical period, this finding is consistent with valproate-activated iPlasticity in the auditory pitch circuitry in humans.
Cholinergic innervation has been known for a long time to regulate critical period plasticity.211 The cholinesterase inhibitor physostigmine was recently demonstrated to induce OD plasticity in the adult mouse visual cortex and to reverse amblyopia in adulthood when given together with patching of the better eye,192 suggesting that cholinesterase inhibitors induce iPlasticity. Since physostigmine has side effects, it would be important to investigate whether other cholinesterase inhibitors with fewer side effects, such as donepezil, which is widely used for dementia, also activate iPlasticity. Indeed, a clinical trial is ongoing to test whether donepezil together with patching of the better eye can be used for the treatment of residual amblyopia after the closure of the natural critical period in humans (https://clinicaltrials.gov/ct2/show/NCT01584076).
Finally, it should be kept in mind that iPlasticity can also be achieved in the visual cortex with purely environmental manipulations, such as environmental enrichment or food restriction.7, 199, 200, 201 iPlasticity induced by fluoxetine and enriched environment closely resemble each other and they both share electrophysiological properties with the naturally occurring critical period. iPlasticity induced by cortical injection of IGF-1 was occluded by the simultaneous exposure to enriched environment; one explanation of this finding is that both treatments share the same core mechanisms.198 However, iPlasticity induced by calorie restriction is not dependent on BDNF.201 Plasticity induced by transcranial magnetic stimulation is more pronounced in BDNF Val66Val subjects than in Met allele carriers, suggesting that iPlasticity may also be involved in the TMS-induced plasticity.212 Clearly, more information is needed about the cellular and molecular mechanisms that govern iPlasticity and to which extent these mechanisms recapitulate those in play during the natural critical periods. Nevertheless, the fact that critical periods are ubiquitous suggests that iPlasticity could be beneficial in the treatment of many neurological and psychiatric disorders, especially when combined with rehabilitation.104
Conclusions
Neurotrophins are regulated by several classes of drugs used in clinical practice. Many of these drugs also induce iPlasticity, a newly recognized pharmacological principle where drugs can be used to directly promote neuronal plasticity. If iPlasticity is induced by drugs that are consumed by millions of people, why has it not been recognized before? One possibility is that iPlasticity is a central mechanism producing the expected clinical effects these drugs, such as mood recovery in the case of antidepressants.104 The disorders being treated by iPlastic drugs, such as depression, may also themselves restrict neuronal plasticity and thereby limit the observable plastic effects. Another potential reason is that although iPlasticity has now been recognized in several species of experimental animals, iPlasticity may not take place in the human brain. Finally, it must be emphasized that iPlastic drugs need to be combined with rehabilitation to guide the plastic networks towards a new function. Such rehabilitation may not be a prominent component of current treatment strategies, but with the advent of smart phones and virtual reality, it is possible to design novel and inexpensive rehabilitation programs that combined with iPlasticity may significantly potentiate the effects of currently available drugs. Such combinations of iPlasticity and rehabilitation could also extend the utility of available drugs into new clinical indications and might have a significant impact on the recovery from, for example, neurological insults. There is already evidence for this.207
References
Katz LC, Shatz CJ . Synaptic activity and the construction of cortical circuits. Science 1996; 274: 1133–1138.
Holtmaat A, Caroni P . Functional and structural underpinnings of neuronal assembly formation in learning. Nat Neurosci 2016; 19: 1553–1562.
Castrén E, Hen R . Neuronal plasticity and antidepressant actions. Trends Neurosci 2013; 36: 259–267.
Hensch TK . Critical period plasticity in local cortical circuits. Nat Rev Neurosci 2005; 6: 877–888.
Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O’leary OF et al. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science 2008; 320: 385–388.
Kobayashi K, Ikeda Y, Sakai A, Yamasaki N, Haneda E, Miyakawa T et al. Reversal of hippocampal neuronal maturation by serotonergic antidepressants. Proc Natl Acad Sci USA 2010; 107: 8434–8439.
Sale A, Berardi N, Maffei L . Environment and brain plasticity: towards an endogenous pharmacotherapy. Physiol Rev 2014; 94: 189–234.
Morishita H, Hensch TK . Critical period revisited: impact on vision. Curr Opin Neurobiol 2008; 18: 101–107.
Poo MM . Neurotrophins as synaptic modulators. Nat Rev Neurosci 2001; 2: 24–32.
Thoenen H . Neurotrophins and neuronal plasticity. Science 1995; 270: 593–598.
Huang EJ, Reichardt LF . Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 2001; 24: 677–736.
Ibanez CF, Andressoo JO . Biology of GDNF and its receptors - relevance for disorders of the central nervous system. Neurobiol Dis 2017; 97: 80–89.
Turner CA, Eren-Kocak E, Inui EG, Watson SJ, Akil H . Dysregulated fibroblast growth factor (fgf) signaling in neurological and psychiatric disorders. Semin Cell Dev Biol 2016; 53: 136–143.
Rauskolb S, Dombert B, Sendtner M . Insulin-like growth factor 1 in diabetic neuropathy and amyotrophic lateral sclerosis. Neurobiol Dis 2016; 97: 103–113.
Levi-Montalcini R, Cohen S . In vitro and in vivo effects of a nerve growth-stimulating agent isolated from snake venom. Proc Natl Acad Sci USA 1956; 42: 695–699.
Barde YA, Edgar D, Thoenen H . Purification of a new neurotrophic factor from mammalian brain. EMBO J 1982; 1: 549–553.
Barde YA . Trophic factors and neuronal survival. Neuron 1989; 2: 1525–1534.
Hohn A, Leibrock J, Bailey K, Barde YA . Identification and characterization of a novel member of the nerve growth factor/brain-derived neurotrophic factor family. Nature 1990; 344: 339–341.
Ernfors P, Ibanez CF, Ebendal T, Olson L, Persson H . Molecular cloning and neurotrophic activities of a protein with structural similarities to nerve growth factor: developmental and topographical expression in the brain. Proc Natl Acad Sci USA 1990; 87: 5454–5458.
Maisonpierre PC, Belluscio L, Squinto S, Ip NY, Furth ME, Lindsay RM et al. Neurotrophin-3: a neurotrophic factor related to ngf and bdnf. Science 1990; 247: 1446–1451.
Hallböök F, Ibanez CF, Persson H . Evolutionary studies of the nerve growth factor family reveal a novel member abundantly expressed in xenopus ovary. Neuron 1991; 6: 845–858.
Berkemeier LR, Winslow JW, Kaplan DR, Nikolics K, Goeddel DV, Rosenthal A . Neurotrophin-5: a novel neurotrophic factor that activates trk and trkb. Neuron 1991; 7: 857–866.
Ip NY, Ibanez CF, Nye SH, Mcclain J, Jones PF, Gies DR et al. Mammalian neurotrophin-4: structure, chromosomal localization, tissue distribution, and receptor specificity. Proc Natl Acad Sci USA 1992; 89: 3060–3064.
Kaplan DR, Hempstead BL, Martin-Zanca D, Chao MV, Parada LF . The trk proto-oncogene product: a signal transducting receptor for nerve growth factor. Science 1991; 252: 554–558.
Klein R, Nanduri V, Jing S, Lamballe F, Tapely P, Bryant S et al. The trk b tyrosine protein kinase is a receptor for brain-derived neurotrophic factor and neurotrophin-3. Cell 1991; 66: 395–403.
Huang EJ, Reichardt LF . Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem 2003; 72: 609–642.
Deinhardt K, Chao MV . Trk receptors. Handb Exp Pharmacol 2014; 220: 103–119.
Chao MV, Bothwell MA, Ross AH, Koprowski H, Lanahan AA, Buck CR et al. Gene transfer and molecular cloning of the human NGF receptor. Science 1986; 232: 518–521.
Radeke MJ, Misko TP, Hsu C, Herzenberg LA, Shooter EM . Gene transfer and molecular cloning of the rat nerve growth factor receptor. Nature 1987; 325: 593–597.
Ibanez CF, Simi A . P75 neurotrophin receptor signaling in nervous system injury and degeneration: paradox and opportunity. Trends Neurosci 2012; 35: 431–440.
Kraemer BR, Yoon SO, Carter BD . The biological functions and signaling mechanisms of the p75 neurotrophin receptor. Handb Exp Pharmacol 2014; 220: 121–164.
Chao MV . Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat.Rev.Neurosci 2003; 4: 299–309.
Lee R, Kermani P, Teng KK, Hempstead BL . Regulation of cell survival by secreted proneurotrophins. Science 2001; 294: 1945–1948.
Hempstead BL . Deciphering proneurotrophin actions. Handb Exp Pharmacol 2014; 220: 17–32.
Thoenen H . The changing scene of neurotrophic factors. Trends Neurosci 1991; 14: 165–170.
Mcallister AK, Katz LC, Lo DC . Neurotrophins and synaptic plasticity. Annu Rev Neurosci 1999; 22: 295–318.
Spillantini MG, Aloe L, Alleva E, Desimone R, Goedert M, Levi-Montalcini R . Nerve growth factor mrna and protein increase in hypothalamus in a mouse model of aggression. Proc Natl Acad Sci USA 1989; 86: 8555–8559.
Gall CM, Isackson PJ . Limbic seizures increase neuronal production of messenger RNA for nerve growth factor. Science 1989; 245: 758–761.
Zafra F, Hengerer B, Leibrock J, Thoenen H, Lindholm D . Activity dependent regulation of bdnf and ngf mrnas in the rat hippocampus is mediated by non-nmda glutamate receptors. EMBO J 1990; 9: 3545–3550.
Lu B, Yokoyama M, Dreyfus CF, Black IB . Depolarizing stimuli regulate nerve growth factor gene expression in cultured hippocampal neurons. Proc Natl Acad Sci USA 1991; 88: 6289–6292.
Isackson PJ, Huntsman MM, Murray KD, Gall CM . Bdnf mrna expression is increased in adult rat forebrain after limbic seizures: temporal pattern of induction distinct from NGF. Neuron 1991; 6: 937–948.
Meyer-Franke A, Wilkinson GA, Kruttgen A, Hu M, Munro E, Hanson MG Jr et al. Depolarization and camp elevation rapidly recruit trkb to the plasma membrane of CNS neurons. Neuron 1998; 21: 681–693.
Du J, Feng L, Yang F, Lu B . Activity- and ca(2+)-dependent modulation of surface expression of brain-derived neurotrophic factor receptors in hippocampal neurons. J Cell Biol 2000; 150: 1423–1434.
Domenici L, Berardi N, Carmignoto G, Vantini G, Maffei L . Nerve growth factor prevents the amblyopic effects of monocular deprivation. Proc Natl Acad Sci USA 1991; 88: 8811–8815.
Maffei L, Berardi N, Domenici L, Parisi V, Pizzorusso T . Nerve growth factor (NGF) prevents the shift in ocular dominance distribution of visual cortical neurons in monocularly deprived rats. J Neurosci 1992; 12: 4651–4662.
Berardi N, Maffei L . From visual experience to visual function: roles of neurotrophins. J Neurobiol 1999; 41: 119–126.
Castrén E, Maya-Vetencourt JF . Neurotrophins as regulators of visual cortcal plasticity. In: Chalupa LM, Berardi N, M C, Galli-Resta L, Pizzorusso T (eds). Cerebral Plasticity. MIT Press: Cambridge, MA, USA, 2011, pp 343–354.
Castrén E, Zafra F, Thoenen H, Lindholm D . Light regulates expression of brain-derived neurotrophic factor mrna in rat visual cortex. Proc Natl Acad Sci USA 1992; 89: 9444–9448.
Cabelli RJ, Hohn A, Shatz CJ . Inhibition of ocular dominance column formation by infusion of nt-4/5 or bdnf. Science 1995; 267: 1662–1666.
Galuske RA, Kim DS, Castrén E, Thoenen H, Singer W . Brain-derived neurotrophic factor reversed experience-dependent synaptic modifications in kitten visual cortex. Eur J Neurosci 1996; 8: 1554–1559.
Cabelli RJ, Shelton DL, Segal RA, Shatz CJ . Blockade of endogenous ligands of trkb inhibits formation of ocular dominance columns. Neuron 1997; 19: 63–76.
Galuske RA, Kim DS, Castrén E, Singer W . Differential effects of neurotrophins on ocular dominance plasticity in developing and adult cat visual cortex. Eur J Neurosci 2000; 12: 3315–3330.
Lodovichi C, Berardi N, Pizzorusso T, Maffei L . Effects of neurotrophins on cortical plasticity: same or different? J Neurosci 2000; 20: 2155–2165.
Cohen-Cory S, Fraser SE . Effects of brain-derived neurotrophic factor on optic axon branching and remodelling in vivo. Nature 1995; 378: 192–196.
Mcallister AK, Lo DC, Katz LC . Neurotrophins regulate dendritic growth in developing visual cortex. Neuron 1995; 15: 791–803.
Mcallister AK, Katz LC, Lo DC . Neurotrophin regulation of cortical dendritic growth requires activity. Neuron 1996; 17: 1057–1064.
Zagrebelsky M, Korte M . Form follows function: Bdnf and its involvement in sculpting the function and structure of synapses. Neuropharmacology 2014; 76: 628–638.
Minichiello L . Trkb signalling pathways in ltp and learning. Nat Rev Neurosci 2009; 10: 850–860.
Park H, Poo MM . Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 2013; 14: 7–23.
Castrén E, Pitkänen M, Sirviö J, Parsadanian A, Lindholm D, Thoenen H et al. The induction of LTP increases BDNF and NGF mrna but decreases nt-3 mrna in the dentate gyrus. NeuroReport 1993; 4: 895–898.
Patterson SL, Grover LM, Schwartzkroin PA, Bothwell M . Neurotrophin expression in rat hippocampal slices: A stimulus paradigm inducing LTP in ca1 evokes increases in BDNF and nt-3 mrnas. Neuron 1992; 9: 1081–1088.
Lohof AM, Ip NY, Poo MM . Potentiation of developing neuromuscular synapses by the neurotrophins nt-3 and bdnf. Nature 1993; 363: 350–353.
Kang H, Schuman EM . Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science 1995; 267: 1658–1662.
Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B . Regulation of synaptic responses to high-frequency stimulation and ltp by neurotrophins in the hippocampus. Nature 1996; 381: 706–709.
Lessmann V, Gottmann K, Heumann R . Bdnf and nt-4/5 enhance glutamatergic synaptic transmission in cultured hippocampal neurones. NeuroReport 1994; 6: 21–25.
Messaoudi E, Bardsen K, Srebro B, Bramham CR . Acute intrahippocampal infusion of bdnf induces lasting potentiation of synaptic transmission in the rat dentate gyrus. J Neurophysiol 1998; 79: 496–499.
Panja D, Kenney JW, D’andrea L, Zalfa F, Vedeler A, Wibrand K et al. Two-stage translational control of dentate gyrus LTP consolidation is mediated by sustained BDNF-TrkB signaling to mnk. Cell Rep 2014; 9: 1430–1445.
Panja D, Bramham CR . Bdnf mechanisms in late ltp formation: a synthesis and breakdown. Neuropharmacology 2014; 76: 664–676.
Kuipers SD, Trentani A, Tiron A, Mao X, Kuhl D, Bramham CR . Bdnf-induced ltp is associated with rapid arc/arg3.1-dependent enhancement in adult hippocampal neurogenesis. Sci Rep 2016; 6: 21222.
Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T . Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci USA 1995; 92: 8856–8860.
Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen S et al. Cleavage of probdnf by tpa/plasmin is essential for long-term hippocampal plasticity. Science 2004; 306: 487–491.
Korte M, Griesbeck O, Gravel C, Carroll P, Staiger V, Thoenen H et al. Virus-mediated gene transfer into hippocampal ca1 region restores long- term potentiation in brain-derived neurotrophic factor mutant mice. Proc Natl Acad Sci USA 1996; 93: 12547–12552.
Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER . Recombinant bdnf rescues deficits in basal synaptic transmission and hippocampal ltp in bdnf knockout mice. Neuron 1996; 16: 1137–1145.
Ninan I, Bath KG, Dagar K, Perez-Castro R, Plummer MR, Lee FS et al. The bdnf val66met polymorphism impairs nmda receptor-dependent synaptic plasticity in the hippocampus. J Neurosci 2010; 30: 8866–8870.
Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A et al. The BDNF val66met polymorphism affects activity-dependent secretion of bdnf and human memory and hippocampal function. Cell 2003; 112: 257–269.
Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG et al. Direct current stimulation promotes bdnf-dependent synaptic plasticity: potential implications for motor learning. Neuron 2010; 66: 198–204.
Bramham CR, Messaoudi E . BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol 2005; 76: 99–125.
Minichiello L, Korte M, Wolfer D, Kuhn R, Unsicker K, Cestari V et al. Essential role for trkb receptors in hippocampus-mediated learning. Neuron 1999; 24: 401–414.
Minichiello L, Calella AM, Medina DL, Bonhoeffer T, Klein R, Korte M . Mechanism of trkb-mediated hippocampal long-term potentiation. Neuron 2002; 36: 121–137.
Koponen E, Voikar V, Riekki R, Saarelainen T, Rauramaa T, Rauvala H et al. Transgenic mice overexpressing the full-length neurotrophin receptor trkb exhibit increased activation of the trkb-plcgamma pathway, reduced anxiety, and facilitated learning. Mol Cell Neurosci 2004; 26: 166–181.
Lu B, Pang PT, Woo NH . The yin and yang of neurotrophin action. Nat Rev Neurosci 2005; 6: 603–614.
Harrington AW, Leiner B, Blechschmitt C, Arevalo JC, Lee R, Morl K et al. Secreted proNGF is a pathophysiological death-inducing ligand after adult CNS injury. Proc Natl Acad Sci USA 2004; 101: 6226–6230.
Shulga A, Thomas-Crusells J, Sigl T, Blaesse A, Mestres P, Meyer M et al. Posttraumatic gaba(a)-mediated [ca2+]I increase is essential for the induction of brain-derived neurotrophic factor-dependent survival of mature central neurons. J Neurosci 2008; 28: 6996–7005.
Mizui T, Ishikawa Y, Kumanogoh H, Lume M, Matsumoto T, Hara T et al. BDNF pro-peptide actions facilitate hippocampal LTD and are altered by the common BDNF polymorphism Val66Met. Proc Natl Acad Sci USA 2015; 112: E3067–E3074.
Guo J, Ji Y, Ding Y, Jiang W, Sun Y, Lu B et al. BDNF pro-peptide regulates dendritic spines via caspase-3. Cell Death Dis 2016; 7: e2264.
Volosin M, Trotter C, Cragnolini A, Kenchappa RS, Light M, Hempstead BL et al. Induction of proneurotrophins and activation of p75ntr-mediated apoptosis via neurotrophin receptor-interacting factor in hippocampal neurons after seizures. J Neurosci 2008; 28: 9870–9879.
Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS et al. Sortilin is essential for prongf-induced neuronal cell death. Nature 2004; 427: 843–848.
Zagrebelsky M, Holz A, Dechant G, Barde YA, Bonhoeffer T, Korte M . The p75 neurotrophin receptor negatively modulates dendrite complexity and spine density in hippocampal neurons. J Neurosci 2005; 25: 9989–9999.
Koshimizu H, Kiyosue K, Hara T, Hazama S, Suzuki S, Uegaki K et al. Multiple functions of precursor bdnf to cns neurons: negative regulation of neurite growth, spine formation and cell survival. Mol Brain 2009; 2: 27.
Deinhardt K, Kim T, Spellman DS, Mains RE, Eipper BA, Neubert TA et al. Neuronal growth cone retraction relies on proneurotrophin receptor signaling through rac. Sci Signal 2011; 4: ra82.
Yang F, Je HS, Ji Y, Nagappan G, Hempstead B, Lu B . Pro-bdnf-induced synaptic depression and retraction at developing neuromuscular synapses. J Cell Biol 2009; 185: 727–741.
Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA et al. Activation of p75ntr by probdnf facilitates hippocampal long-term depression. Nat Neurosci 2005; 8: 1069–1077.
Martinowich K, Schloesser RJ, Lu Y, Jimenez DV, Paredes D, Greene JS et al. Roles of p75(ntr), long-term depression, and cholinergic transmission in anxiety and acute stress coping. Biol Psychiatry 2012; 71: 75–83.
Winnubst J, Cheyne JE, Niculescu D, Lohmann C . Spontaneous activity drives local synaptic plasticity in vivo. Neuron 2015; 87: 399–410.
Deppmann CD, Mihalas S, Sharma N, Lonze BE, Niebur E, Ginty DD . A model for neuronal competition during development. Science 2008; 320: 369–373.
Singh KK, Park KJ, Hong EJ, Kramer BM, Greenberg ME, Kaplan DR et al. Developmental axon pruning mediated by bdnf-p75ntr-dependent axon degeneration. Nat Neurosci 2008; 11: 649–658.
Je HS, Yang F, Ji Y, Nagappan G, Hempstead BL, Lu B . Role of pro-brain-derived neurotrophic factor (probdnf) to mature bdnf conversion in activity-dependent competition at developing neuromuscular synapses. Proc Natl Acad Sci USA 2012; 109: 15924–15929.
Je HS, Yang F, Ji Y, Potluri S, Fu XQ, Luo ZG et al. Probdnf and mature bdnf as punishment and reward signals for synapse elimination at mouse neuromuscular junctions. J Neurosci 2013; 33: 9957–9962.
Thoenen H, Sendtner M . Neurotrophins: from enthusiastic expectations through sobering experiences to rational therapeutic approaches. Nat Neurosci 2002; 5 (suppl): 1046–1050.
Bartus RT, Johnson EMJ . Clinical tests of neurotrophic factors for human neurodegenerative diseases, part 1: where have we been and what have we learned. Neurobiol Dis 2016; 97: 156–168.
Josephy-Hernandez S, Jmaeff S, Pirvulescu I, Aboulkassim T, Saragovi HU . Neurotrophin receptor agonists and antagonists as therapeutic agents: an evolving paradigm. Neurobiol Dis 2016; 97: 139–155.
Duman RS, Heninger GR, Nestler EJ . A molecular and cellular theory of depression. Arch Gen Psychiatry 1997; 54: 597–606.
Autry AE, Monteggia LM . Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev 2012; 64: 238–258.
Castrén E . Neuronal network plasticity and recovery from depression. JAMA Psychiatry 2013; 70: 983–989.
Lindholm JS, Castrén E . Mice with altered bdnf signaling as models for mood disorders and antidepressant effects. Front Behav Neurosci 2014; 8: 143.
Nibuya M, Morinobu S, Duman RS . Regulation of bdnf and trkb mrna in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J.Neurosci 1995; 15: 7539–7547.
Dias BG, Banerjee SB, Duman RS, Vaidya VA . Differential regulation of brain derived neurotrophic factor transcripts by antidepressant treatments in the adult rat brain. Neuropharmacology 2003; 45: 553–563.
Russo-Neustadt A, Beard RC, Cotman CW . Exercise, antidepressant medications, and enhanced brain derived neurotrophic factor expression. Neuropsychopharmacology 1999; 21: 679–682.
Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ . Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci 2006; 9: 519–525.
Sairanen M, O’leary OF, Knuuttila JE, Castrén E . Chronic antidepressant treatment selectively increases expression of plasticity-related proteins in the hippocampus and medial prefrontal cortex of the rat. Neuroscience 2007; 144: 368–374.
Wibrand K, Messaoudi E, Havik B, Steenslid V, Lovlie R, Steen VM et al. Identification of genes co-upregulated with arc during bdnf-induced long-term potentiation in adult rat dentate gyrus in vivo. Eur J Neurosci 2006; 23: 1501–1511.
Alme MN, Wibrand K, Dagestad G, Bramham CR . Chronic fluoxetine treatment induces brain region-specific upregulation of genes associated with bdnf-induced long-term potentiation. Neural Plast 2007; 2007: 26496.
Russo-Neustadt A, Ha T, Ramirez R, Kesslak JP . Physical activity-antidepressant treatment combination: impact on brain-derived neurotrophic factor and behavior in an animal model. Behav Brain Res 2001; 120: 87–95.
Altar CA, Whitehead RE, Chen R, Wortwein G, Madsen TM . Effects of electroconvulsive seizures and antidepressant drugs on brain-derived neurotrophic factor protein in rat brain. Biol Psychiatry 2003; 54: 703–709.
Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, Macdonald E et al. Activation of the trkb neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci 2003; 23: 349–357.
Rantamäki T, Hendolin P, Kankaanpaa A, Mijatovic J, Piepponen P, Domenici E et al. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor trkb and induce phospholipase-cgamma signaling pathways in mouse brain. Neuropsychopharmacology 2007; 32: 2152–2162.
Rantamäki T, Vesa L, Antila H, Di Lieto A, Tammela P, Schmitt A et al. Antidepressant drugs transactivate trkb neurotrophin receptors in the adult rodent brain independently of bdnf and monoamine transporter blockade. PLoS One 2011; 6: e20567.
Chen B, Dowlatshahi D, Macqueen GM, Wang JF, Young LT . Increased hippocampal bdnf immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry 2001; 50: 260–265.
Rocha RB, Dondossola ER, Grande AJ, Colonetti T, Ceretta LB, Passos IC et al. Increased bdnf levels after electroconvulsive therapy in patients with major depressive disorder: a meta-analysis study. J Psychiatr Res 2016; 83: 47–53.
Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN . Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase b in postmortem brain of suicide subjects. Arch Gen Psychiatry 2003; 60: 804–815.
Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T et al. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci USA 2004; 101: 10827–10832.
Ibarguen-Vargas Y, Surget A, Vourc’h P, Leman S, Andres CR, Gardier AM et al. Deficit in bdnf does not increase vulnerability to stress but dampens antidepressant-like effects in the unpredictable chronic mild stress. Behav Brain Res 2009; 202: 245–251.
Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM . Antidepressant-like effect of brain-derived neurotrophic factor (bdnf). Pharmacol Biochem Behav 1997; 56: 131–137.
Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS . Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci 2002; 22: 3251–3261.
Saarelainen T, Vaittinen S, Castrén E . Trkb-receptor activation contributes to the kainate-induced increase in bdnf mrna synthesis. Cell Mol Neurobiol 2001; 21: 429–435.
Tuvikene J, Pruunsild P, Orav E, Esvald EE, Timmusk T . Ap-1 transcription factors mediate bdnf-positive feedback loop in cortical neurons. J Neurosci 2016; 36: 1290–1305.
Koponen E, Rantamäki T, Voikar V, Saarelainen T, Macdonald E, Castrén E . Enhanced bdnf signaling is associated with an antidepressant-like behavioral response and changes in brain monoamines. Cell Mol Neurobiol 2005; 25: 973–980.
Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG et al. Trkb regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron 2008; 59: 399–412.
Arango-Lievano M, Lambert WM, Bath KG, Garabedian MJ, Chao MV, Jeanneteau F . Neurotrophic-priming of glucocorticoid receptor signaling is essential for neuronal plasticity to stress and antidepressant treatment. Proc Natl Acad Sci USA 2015; 112: 15737–15742.
Lambert WM, Xu CF, Neubert TA, Chao MV, Garabedian MJ, Jeanneteau FD . Brain-derived neurotrophic factor signaling rewrites the glucocorticoid transcriptome via glucocorticoid receptor phosphorylation. Mol Cell Biol 2013; 33: 3700–3714.
Ming GL, Song H . Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 2011; 70: 687–702.
Malberg JE, Eisch AJ, Nestler EJ, Duman RS . Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 2000; 20: 9104–9110.
Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003; 301: 805–809.
Sahay A, Hen R . Adult hippocampal neurogenesis in depression. Nat Neurosci 2007; 10: 1110–1115.
Wu X, Castrén E . Co-treatment with diazepam prevents the effects of fluoxetine on the proliferation and survival of hippocampal dentate granule cells. Biol Psychiatry 2009; 66: 5–8.
David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 2009; 62: 479–493.
Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA et al. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry 2009; 14: 764–773, 739.
Sairanen M, Lucas G, Ernfors P, Castrén M, Castrén E . Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci 2005; 25: 1089–1094.
Wang JW, David DJ, Monckton JE, Battaglia F, Hen R . Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci 2008; 28: 1374–1384.
Hajszan T, Dow A, Warner-Schmidt JL, Szigeti-Buck K, Sallam NL, Parducz A et al. Remodeling of hippocampal spine synapses in the rat learned helplessness model of depression. Biol Psychiatry 2009; 65: 392–400.
Chen JL, Lin WC, Cha JW, So PT, Kubota Y, Nedivi E . Structural basis for the role of inhibition in facilitating adult brain plasticity. Nat Neurosci 2011; 14: 587–594.
Stewart CA, Reid IC . Repeated ecs and fluoxetine administration have equivalent effects on hippocampal synaptic plasticity. Psychopharmacology (Berl) 2000; 148: 217–223.
Karpova NN, Pickenhagen A, Lindholm J, Tiraboschi E, Kulesskaya N, Agustsdottir A et al. Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science 2011; 334: 1731–1734.
Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K . High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science 2007; 317: 819–823.
Stepan J, Hladky F, Uribe A, Holsboer F, Schmidt MV, Eder M . High-speed imaging reveals opposing effects of chronic stress and antidepressants on neuronal activity propagation through the hippocampal trisynaptic circuit. Front Neural Circuits 2015; 9: 70.
Zarate CAJ, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA et al. A randomized trial of an n-methyl-d-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 2006; 63: 856–864.
Abdallah CG, Sanacora G, Duman RS, Krystal JH . Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med 2015; 66: 509–523.
Duman RS, Aghajanian GK, Sanacora G, Krystal JH . Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 2016; 22: 238–249.
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M et al. Mtor-dependent synapse formation underlies the rapid antidepressant effects of nmda antagonists. Science 2010; 329: 959–964.
Keilhoff G, Bernstein HG, Becker A, Grecksch G, Wolf G . Increased neurogenesis in a rat ketamine model of schizophrenia. Biol Psychiatry 2004; 56: 317–322.
Soumier A, Carter RM, Schoenfeld TJ, Cameron HA . New hippocampal neurons mature rapidly in response to ketamine but are not required for its acute antidepressant effects on neophagia in rats. eNeuro 2016; 3: e0116-15.2016.
Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK . Brain-derived neurotrophic factor val66met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry 2012; 71: 996–1005.
Laje G, Lally N, Mathews D, Brutsche N, Chemerinski A, Akula N et al. Brain-derived neurotrophic factor val66met polymorphism and antidepressant efficacy of ketamine in depressed patients. Biol Psychiatry 2012; 72: e27–e28.
Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS . Bdnf release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol 2014; 18; doi: 10.1093/ijnp/pyu033.
Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI et al. Nmdar inhibition-independent antidepressant actions of ketamine metabolites. Nature 2016; 533: 481–486.
Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF et al. Nmda receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 2011; 475: 91–95.
Dagestad G, Kuipers SD, Messaoudi E, Bramham CR . Chronic fluoxetine induces region-specific changes in translation factor eif4e and eef2 activity in the rat brain. Eur J Neurosci 2006; 23: 2814–2818.
Lindholm JS, Autio H, Vesa L, Antila H, Lindemann L, Hoener MC et al. The antidepressant-like effects of glutamatergic drugs ketamine and ampa receptor potentiator ly 451646 are preserved in bdnf(+)/(-) heterozygous null mice. Neuropharmacology 2012; 62: 391–397.
Brunkhorst R, Vutukuri R, Pfeilschifter W . Fingolimod for the treatment of neurological diseases-state of play and future perspectives. Front Cell Neurosci 2014; 8: 283.
Deogracias R, Yazdani M, Dekkers MP, Guy J, Ionescu MC, Vogt KE et al. Fingolimod, a sphingosine-1 phosphate receptor modulator, increases bdnf levels and improves symptoms of a mouse model of rett syndrome. Proc Natl Acad Sci USA 2012; 109: 14230–14235.
Miguez A, Garcia-Diaz Barriga G, Brito V, Straccia M, Giralt A, Gines S et al. Fingolimod (fty720) enhances hippocampal synaptic plasticity and memory in huntington’s disease by preventing p75ntr up-regulation and astrocyte-mediated inflammation. Hum Mol Genet 2015; 24: 4958–4970.
Di Nuzzo L, Orlando R, Tognoli C, Di Pietro P, Bertini G, Miele J et al. Antidepressant activity of fingolimod in mice. Pharmacol Res Perspect 2015; 3: e00135.
Lee FS, Rajagopal R, Chao MV . Distinctive features of trk neurotrophin receptor transactivation by g protein-coupled receptors. Cytokine Growth Factor Rev 2002; 13: 11–17.
Lee FS, Chao MV . Activation of trk neurotrophin receptors in the absence of neurotrophins. Proc Natl Acad Sci USA 2001; 98: 3555–3560.
Lee FS, Rajagopal R, Kim AH, Chang PC, Chao MV . Activation of trk neurotrophin receptor signaling by pituitary adenylate cyclase-activating polypeptides. J Biol Chem 2002; 277: 9096–9102.
Wiese S, Jablonka S, Holtmann B, Orel N, Rajagopal R, Chao MV et al. Adenosine receptor a2a-r contributes to motoneuron survival by transactivating the tyrosine kinase receptor trkb. Proc Natl Acad Sci USA 2007; 104: 17210–17215.
Jeanneteau F, Garabedian MJ, Chao MV . Activation of trk neurotrophin receptors by glucocorticoids provides a neuroprotective effect. Proc Natl Acad Sci USA 2008; 105: 4862–4867.
Korpi ER, Den Hollander B, Farooq U, Vashchinkina E, Rajkumar R, Nutt DJ et al. Mechanisms of action and persistent neuroplasticity by drugs of abuse. Pharmacol Rev 2015; 67: 872–1004.
Lüscher C, Malenka RC . Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron 2011; 69: 650–663.
Dong Y, Nestler EJ . The neural rejuvenation hypothesis of cocaine addiction. Trends Pharmacol Sci 2014; 35: 374–383.
Ungless MA, Whistler JL, Malenka RC, Bonci A . Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 2001; 411: 583–587.
Huang YH, Lin Y, Mu P, Lee BR, Brown TE, Wayman G et al. In vivo cocaine experience generates silent synapses. Neuron 2009; 63: 40–47.
Lee BR, Ma YY, Huang YH, Wang X, Otaka M, Ishikawa M et al. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci 2013; 16: 1644–1651.
Russo SJ, Mazei-Robison MS, Ables JL, Nestler EJ . Neurotrophic factors and structural plasticity in addiction. Neuropharmacology 2009; 56: 73–82.
Lobo MK, Covington HE, Chaudhury D, Friedman AK, Sun H, Damez-Werno D et al. Cell type-specific loss of bdnf signaling mimics optogenetic control of cocaine reward. Science 2010; 330: 385–390.
Graham DL, Edwards S, Bachtell RK, Dileone RJ, Rios M, Self DW . Dynamic bdnf activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci 2007; 10: 1029–1037.
Graham DL, Krishnan V, Larson EB, Graham A, Edwards S, Bachtell RK et al. Tropomyosin-related kinase b in the mesolimbic dopamine system: region-specific effects on cocaine reward. Biol Psychiatry 2009; 65: 696–701.
Koo JW, Mazei-Robison MS, Chaudhury D, Juarez B, Laplant Q, Ferguson D et al. Bdnf is a negative modulator of morphine action. Science 2012; 338: 124–128.
Koskela M, Back S, Voikar V, Richie CT, Domanskyi A, Harvey BK et al. Update of neurotrophic factors in neurobiology of addiction and future directions. Neurobiol Dis 2016; 97: 189–200.
Wiesel TN . Postnatal development of the visual cortex and the influence of environment. Nature 1982; 299: 583–591.
Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E . Increased cortical representation of the fingers of the left hand in string players. Science 1995; 270: 305–307.
Kuhl PK . Brain mechanisms in early language acquisition. Neuron 2010; 67: 713–727.
Leppänen JM, Nelson CA . Tuning the developing brain to social signals of emotions. Nat Rev Neurosci 2009; 10: 37–47.
Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF . Local gaba circuit control of experience-dependent plasticity in developing visual cortex. Science 1998; 282: 1504–1508.
Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF et al. Bdnf regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 1999; 98: 739–755.
Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK . Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci 2010; 30: 14964–14971.
Wang D, Fawcett J . The perineuronal net and the control of cns plasticity. Cell Tissue Res 2012; 349: 147–160.
Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L . Reactivation of ocular dominance plasticity in the adult visual cortex. Science 2002; 298: 1248–1251.
Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN et al. Chondroitinase abc promotes functional recovery after spinal cord injury. Nature 2002; 416: 636–640.
Gogolla N, Caroni P, Luthi A, Herry C . Perineuronal nets protect fear memories from erasure. Science 2009; 325: 1258–1261.
Scali M, Begenisic T, Mainardi M, Milanese M, Bonifacino T, Bonanno G et al. Fluoxetine treatment promotes functional recovery in a rat model of cervical spinal cord injury. Sci Rep 2013; 3: 2217.
Morishita H, Miwa JM, Heintz N, Hensch TK . Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science 2010; 330: 1238–1240.
Maya Vetencourt JF, Tiraboschi E, Spolidoro M, Castrén E, Maffei L . Serotonin triggers a transient epigenetic mechanism that reinstates adult visual cortex plasticity in rats. Eur J Neurosci 2011; 33: 49–57.
Putignano E, Lonetti G, Cancedda L, Ratto G, Costa M, Maffei L et al. Developmental downregulation of histone posttranslational modifications regulates visual cortical plasticity. Neuron 2007; 53: 747–759.
Silingardi D, Scali M, Belluomini G, Pizzorusso T . Epigenetic treatments of adult rats promote recovery from visual acuity deficits induced by long-term monocular deprivation. Eur J Neurosci 2010; 31: 2185–2192.
Yang EJ, Lin EW, Hensch TK . Critical period for acoustic preference in mice. Proc Natl Acad Sci USA 2012; 109: 17213–17220.
Gervain J, Vines BW, Chen LM, Seo RJ, Hensch TK, Werker JF et al. Valproate reopens critical-period learning of absolute pitch. Front Syst Neurosci 2013; 7: 102.
Maya-Vetencourt JF, Baroncelli L, Viegi A, Tiraboschi E, Castrén E, Cattaneo A et al. Igf-1 restores visual cortex plasticity in adult life by reducing local gaba levels. Neural Plast 2012; 2012: 250421.
Sale A, Maya Vetencourt JF, Medini P, Cenni MC, Baroncelli L, De Pasquale R et al. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat Neurosci 2007; 10: 679–681.
Greifzu F, Pielecka-Fortuna J, Kalogeraki E, Krempler K, Favaro PD, Schluter OM et al. Environmental enrichment extends ocular dominance plasticity into adulthood and protects from stroke-induced impairments of plasticity. Proc Natl Acad Sci USA 2014; 111: 1150–1155.
Spolidoro M, Baroncelli L, Putignano E, Maya-Vetencourt JF, Viegi A, Maffei L . Food restriction enhances visual cortex plasticity in adulthood. Nat Commun 2011; 2: 320.
Mcewen BS, Chattarji S, Diamond DM, Jay TM, Reagan LP, Svenningsson P et al. The neurobiological properties of tianeptine (stablon): from monoamine hypothesis to glutamatergic modulation. Mol Psychiatry 2010; 15: 237–249.
Popova D, Ágústsdóttir A, Lindholm J, Mazulis U, Akamine Y, Castrén E et al. Combination of fluoxetine and extinction treatments forms a unique synaptic protein profile that correlates with long-term fear reduction in adult mice. Eur Neuropsychopharmacol 2014; 24: 1162–1174.
Hagihara H, Takao K, Walton NM, Matsumoto M, Miyakawa T . Immature dentate gyrus: an endophenotype of neuropsychiatric disorders. Neural Plast 2013; 2013: 318596.
Acler M, Robol E, Fiaschi A, Manganotti P . A double blind placebo rct to investigate the effects of serotonergic modulation on brain excitability and motor recovery in stroke patients. J Neurol 2009; 256: 1152–1158.
Jorge RE, Acion L, Moser D, Adams HPJ, Robinson RG . Escitalopram and enhancement of cognitive recovery following stroke. Arch Gen Psychiatry 2010; 67: 187–196.
Chollet F, Tardy J, Albucher JF, Thalamas C, Berard E, Lamy C et al. Fluoxetine for motor recovery after acute ischaemic stroke (flame): a randomised placebo-controlled trial. Lancet Neurol 2011; 10: 123–130.
Normann C, Schmitz D, Furmaier A, Doing C, Bach M . Long-term plasticity of visually evoked potentials in humans is altered in major depression. Biol Psychiatry 2007; 62: 373–380.
Vialou V, Feng J, Robison AJ, Nestler EJ . Epigenetic mechanisms of depression and antidepressant action. Annu Rev Pharmacol Toxicol 2013; 53: 59–87.
Karpova NN . Role of bdnf epigenetics in activity-dependent neuronal plasticity. Neuropharmacology 2014; 76 (Pt C): 709–718.
Bear MF, Singer W . Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature 1986; 320: 172–176.
Chang WH, Bang OY, Shin YI, Lee A, Pascual-Leone A, Kim YH . Bdnf polymorphism and differential rtms effects on motor recovery of stroke patients. Brain Stimul 2014; 7: 553–558.
Acknowledgements
We thank the Trophin laboratory members for stimulating discussions, Jussi Kupari for help with the figures and Adrian Goldman and Johanna Ratia for language revision. The original research in our laboratory has been supported by the ERC grant No 322742 – iPLASTICITY, the Sigrid Jusélius foundation and Academy of Finland grants # 294710 and # 307416.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
PowerPoint slides
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Castrén, E., Antila, H. Neuronal plasticity and neurotrophic factors in drug responses. Mol Psychiatry 22, 1085–1095 (2017). https://doi.org/10.1038/mp.2017.61
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2017.61
This article is cited by
-
Lasting dynamic effects of the psychedelic 2,5-dimethoxy-4-iodoamphetamine ((±)-DOI) on cognitive flexibility
Molecular Psychiatry (2024)
-
Serotonergic psychedelic 5-MeO-DMT alters plasticity-related gene expression and generates anxiolytic effects in stressed mice
Molecular Psychiatry (2024)
-
Ethopharmacological evaluation of antidepressant-like effect of serotonergic psychedelics in C57BL/6J male mice
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
The impact of purine nucleosides on neuroplasticity in the adult brain
Purinergic Signalling (2024)
-
Mutation in the TRKB Cholesterol Recognition Site that blocks Antidepressant Binding does not Influence the Basal or BDNF-Stimulated Activation of TRKB
Cellular and Molecular Neurobiology (2024)