Abstract
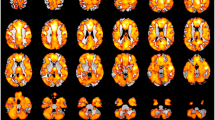
Psychosis commonly develops in adolescence or early adulthood. Youths at clinical high risk (CHR) for psychosis exhibit similar, subtle symptoms to those with schizophrenia (SZ). Malfunctioning neurotransmitter systems, such as glutamate, are implicated in the disease progression of psychosis. Yet, in vivo imaging techniques for measuring glutamate across the cortex are limited. Here, we use a novel 7 Tesla MRI glutamate imaging technique (GluCEST) to estimate changes in glutamate levels across cortical and subcortical regions in young healthy individuals and ones on the psychosis spectrum. Individuals on the psychosis spectrum (PS; n=19) and healthy young individuals (HC; n=17) underwent MRI imaging at 3 and 7 T. At 7 T, a single slice GluCEST technique was used to estimate in vivo glutamate. GluCEST contrast was compared within and across the subcortex, frontal, parietal and occipital lobes. Subcortical (χ2 (1)=4.65, P=0.031) and lobular (χ2 (1)=5.17, P=0.023) GluCEST contrast levels were lower in PS compared with HC. Abnormal GluCEST contrast levels were evident in both CHR (n=14) and SZ (n=5) subjects, and correlated differentially, across regions, with clinical symptoms. Our findings describe a pattern of abnormal brain neurochemistry early in the course of psychosis. Specifically, CHR and young SZ exhibit diffuse abnormalities in GluCEST contrast attributable to a major contribution from glutamate. We suggest that neurochemical profiles of GluCEST contrast across cortex and subcortex may be considered markers of early psychosis. GluCEST methodology thus shows promise to further elucidate the progression of the psychosis disease state.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Casey BJ, Oliveri ME, Insel T . A neurodevelopmental perspective on the research domain criteria (RDoC) framework. Biol Psychiatry 2014; 76: 350–353.
Insel TR . Rethinking schizophrenia. Nature 2010; 468: 187–193.
Rapoport JL, Giedd JN, Gogtay N . Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry 2012; 17: 1228–1238.
Gur RE, Turetsky BI, Cowell PE, Finkelman C, Maany V, Grossman RI et al. Temporolimbic volume reductions in schizophrenia. Arch Gen Psychiatry 2000; 57: 769–775.
Bora E, Fornito A, Radua J, Walterfang M, Seal M, Wood SJ et al. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res 2011; 127: 46–57.
Selemon LD, Goldman-Rakic PS . The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry 1999; 45: 17–25.
Laruelle M, Kegeles LS, Abi-Dargham A . Glutamate, dopamine, and schizophrenia. Ann N Y Acad Sci 2006; 1003: 138–158.
Poels EMP, Kegeles LS, Kantrowitz JT, Slifstein M, Javitt DC, Lieberman JA et al. Imaging glutamate in schizophrenia: review of findings and implications for drug discovery. Mol Psychiatry 2014; 19: 20–29.
Marsman A, van den Heuvel MP, Klomp DWJ, Kahn RS, Luijten PR, Pol HEH . Glutamate in schizophrenia: a focused review and meta-analysis of 1H-MRS studies. Schizophr Bull 2013; 39: 120–129.
Remington G, Agid O, Foussias G . Schizophrenia as a disorder of too little dopamine: implications for symptoms and treatment. Expert Rev Neurother 2011; 11: 589–607.
Lewis DA, Moghaddam B . Cognitive dysfunction in schizophrenia: convergence of {gamma}-aminobutyric acid and glutamate alterations. Arch Neurol 2006; 63: 1372.
Coyle JT . Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol 2006; 26: 363–382.
Moghaddam B . Targeting metabotropic glutamate receptors for treatment of the cognitive symptoms of schizophrenia. Psychopharmacology 2004; 174: 39–44.
Satterthwaite TD, Wolf DH, Calkins ME, Vandekar SN, Erus G, Ruparel K et al. Structural brain abnormalities in youth with psychosis spectrum symptoms. JAMA Psychiatry 2016; 73: 515–524.
Egerton A, Stone JM, Chaddock CA, Barker GJ, Bonoldi I, Howard RM et al. Relationship between brain glutamate levels and clinical outcome in individuals at ultra high risk of psychosis. Neuropsychopharmacology 39: 2891–2899 2014.
Allen P, Chaddock CA, Egerton A, Howes OD, Barker G, Bonoldi I et al. Functional outcome in people at high risk for psychosis predicted by thalamic glutamate levels and prefronto-striatal activation. Schizophr Bull 2015; 41: 429–439.
Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry 2008; 65: 28–37.
Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry 2012; 69: 220.
Merritt K, Egerton A, Kempton MJ, Taylor MJ, McGuire PK . Nature of glutamate alterations in schizophrenia: a meta-analysis of proton magnetic resonance spectroscopy studies. JAMA Psychiatry 2016; 73: 665–674.
de la Fuente-Sandoval C, León-Ortiz P, Azcárraga M, Stephano S, Favila R, Díaz-Galvis L et al. Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA Psychiatry 2013; 70: 1057–1066.
Kegeles LS, Mao X, Stanford AD, Girgis R, Ojeil N, Xu X et al. Elevated prefrontal cortex {gamma}-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry 2012; 69: 449–459..
Wijtenburg S, Wright S, Korenic S, Gaston F, Ndubuizu N, Chiappelli J et al. Altered glutamate and regional cerebral blood flow levels in schizophrenia: a 1H-MRS and pCASL Study. Neuropsychopharmacology 2016; 42: 562–571.
Theberge J, Bartha R, Drost DJ, Menon RS, Malla A, Takhar J et al. Glutamate and glutamine measured with 4.0T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry 2002; 159: 1944–1946.
Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH . Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology 2007; 32: 1888–1902.
Goff DC, Coyle JT . The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry 2001; 158: 1367–1377.
Tsai G, Coyle JT . Glutamatergic mechanisms in schizophrenia. Annu Rev Pharmacol Toxicol 2002; 42: 165–179.
Treen D, Batlle S, Mollà L, Forcadell E, Chamorro J, Bulbena A et al. Are there glutamate abnormalities in subjects at high risk mental state for psychosis? A review of the evidence. Schizophr Res 2016; 171: 166–175.
Uhl I, Mavrogiorgou P, Norra C, Forstreuter F, Scheel M, Witthaus H et al. 1H-MR spectroscopy in ultra-high risk and first episode stages of schizophrenia. J Psychiatr Res 2011; 45: 1135–1139.
Stone JM, Day F, Tsagaraki H, Valli I, McLean MA, Lythgoe DJ et al. Glutamate dysfunction in people with prodromal symptoms of psychosis: relationship to gray matter volume. Biol Psychiatry 2009; 66: 533–539.
Keshavan MS, Dick RM, Diwadkar VA, Montrose DM, Prasad KM, Stanley JA . Striatal metabolic alterations in non-psychotic adolescent offspring at risk for schizophrenia: a 1 H spectroscopy study. Schizophr Res 2009; 115: 88–93.
Tibbo P, Hanstock C, Valiakalayil A, Allen P . 3-T proton MRS investigation of glutamate and glutamine in adolescents at high genetic risk for schizophrenia. Am J Psychiatry 2004; 161: 1116–1118.
Fusar-Poli P, Stone JM, Broome MR, Valli I, Mechelli A, McLean MA et al. Thalamic glutamate levels as a predictor of cortical response during executive functioning in subjects at high risk for psychosis. Arch Gen Psychiatry 2011; 68: 881.
de la Fuente-Sandoval C, León-Ortiz P, Azcárraga M, Favila R, Stephano S, Graff-Guerrero A . Striatal glutamate and the conversion to psychosis: a prospective 1 H-MRS imaging study. Int J Neuropsychopharmacol 2013; 16: 471–475.
de la Fuente-Sandoval C, Leon-Ortiz P, Favila R, Stephano S, Mamo D, Ramirez-Bermudez J et al. Higher levels of glutamate in the associative-striatum of subjects with prodromal symptoms of schizophrenia and patients with first-episode psychosis. Neuropsychopharmacology 2011; 36: 1781–1791.
Tandon N, Bolo NR, Sanghavi K, Mathew IT, Francis AN, Stanley JA et al. Brain metabolite alterations in young adults at familial high risk for schizophrenia using proton magnetic resonance spectroscopy. Schizophr Res 2013; 148: 59–66.
Yoo SY, Yeon S, Choi C-H, Kang D-H, Lee J-M, Shin NY et al. Proton magnetic resonance spectroscopy in subjects with high genetic risk of schizophrenia: investigation of anterior cingulate, dorsolateral prefrontal cortex and thalamus. Schizophr Res 2009; 111: 86–93.
Purdon SE, Valiakalayil A, Hanstock CC, Seres P, Tibbo P . Elevated 3T proton MRS glutamate levels associated with poor continuous performance test (CPT-0X) scores and genetic risk for schizophrenia. Schizophr Res 2008; 99: 218–224.
Natsubori T, Inoue H, Abe O, Takano Y, Iwashiro N, Aoki Y et al. Reduced frontal glutamate+ glutamine and N-acetylaspartate levels in patients with chronic schizophrenia but not in those at clinical high risk for psychosis or with first-episode schizophrenia. Schizophr Bull 2014; 40: 1128–1139.
Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron 2013; 78: 81–93.
Cai K, Haris M, Singh A, Kogan F, Greenberg JH, Hariharan H et al. Magnetic resonance imaging of glutamate. Nat Med 2012; 18: 302–306.
Cai K, Singh A, Roalf DR, Nanga RPR, Haris M, Hariharan H et al. Mapping glutamate in subcortical brain structures using high‐resolution GluCEST MRI. NMR Biomed 2013; 26: 1278–1284.
Kogan F, Singh A, Debrosse C, Haris M, Cai K, Nanga RP et al. Imaging of glutamate in the spinal cord using GluCEST. Neuroimage 2013; 77: 262–267.
van Zijl P, Yadav NN . Chemical exchange saturation transfer (CEST): what is in a name and what isn't? Magn Reson Med 2011; 65: 927–948.
Sherry AD, Woods M . Chemical exchange saturation transfer contrast agents for magnetic resonance imaging. Ann Rev Biomed Eng 2008; 10: 391.
Zhou J, van Zijl PC . Chemical exchange saturation transfer imaging and spectroscopy. Prog Nuc Magn Reson Spectrosc 2006; 48: 109–136.
Henkelman R, Stanisz G, Graham S . Magnetization transfer in MRI: a review. NMR Biomed 2001; 14: 57–64.
Haris M, Nath K, Cai K, Singh A, Crescenzi R, Kogan F et al. Imaging of glutamate neurotransmitter alterations in Alzheimer's disease. NMR Biomed 2013; 26: 386–391.
Crescenzi R, DeBrosse C, Nanga RPR, Reddy S, Haris M, Hariharan H et al. In vivo measurement of glutamate loss is associated with synapse loss in a mouse model of tauopathy. Neuroimage 2014; 101: 185–192.
Pépin J, Francelle L, Carrillo-de Sauvage M-A, de Longprez L, Gipchtein P, Cambon K et al. In vivo imaging of brain glutamate defects in a knock-in mouse model of Huntington's disease. Neuroimage 2016; 139: 53–64.
Davis KA, Nanga RPR, Das S, Chen SH, Hadar PN, Pollard JR et al. Glutamate imaging (GluCEST) lateralizes epileptic foci in nonlesional temporal lobe epilepsy. Sci Transl Med 2015; 7: 309ra161–309ra161.
Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36: 980–988.
First MB, Spitzer RL, Gibbon M, Williams JBW . Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). Biometrics Research. New York State Psychiatric Institute: New York, NY, USA, 2002.
McGlashan TH, Miller TJ, Woods SW, Hoffman RE, Davidson L . Instrument for the assessment of prodromal symptoms and states. In: Miller TJ, Mednick SA, et al. (eds). Early Intervention in Psychotic Disorders. Kluwer Academic Publishers: Amsterdam, Netherlands, 2001, pp 135–149.
Overall JE, Gorham DR . The brief psychiatric rating scale. Psychol Rep 1962; 10: 799–812.
Andreasen NC . The Scale for the Assessment of Negative Symptoms (SANS). University of Iowa: Iowa City, IA, USA, 1984.
Andreasen NC . The Scale for the Assessment of Positive Symptoms (SAPS). University of Iowa: Iowa City, IA, USA, 1984.
Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM et al. Symptom assessment in schizophrenic prodromal states. Psychiatr Q 1999; 70: 273–287.
Roalf DR, Quarmley M, Elliott MA, Satterthwaite TD, Vandekar SN, Ruparel K et al. The impact of quality assurance assessment on diffusion tensor imaging outcomes in a large-scale population-based cohort. Neuroimage 2016; 125: 903–919.
Gur RE, Turetsky BI, Bilker WB, Gur RC . Reduced gray matter volume in schizophrenia. Arch Gen Psychiatry 1999; 56: 905.
Karlsgodt KH, Niendam TA, Bearden CE, Cannon TD . White matter integrity and prediction of social and role functioning in subjects at ultra-high risk for psychosis. Biol Psychiatry 2009; 66: 562–569.
Roalf DR, Gur RE, Verma R, Parker WA, Quarmley M, Ruparel K et al. White matter microstructure in schizophrenia: associations to neurocognition and clinical symptomatology. Schizophr Res 2015; 161: 42–49.
Provencher SW . Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993; 30: 672–679.
Brugger S, Davis JM, Leucht S, Stone JM . Proton magnetic resonance spectroscopy and illness stage in schizophrenia: a systematic review and meta-analysis. Biol Psychiatry 2011; 69: 495–503.
Falkenberg LE, Westerhausen R, Specht K, Hugdahl K . Resting-state glutamate level in the anterior cingulate predicts blood-oxygen level-dependent response to cognitive control. Proc Natl Acad Sci USA 2012; 109: 5069–5073.
Stanley JA, Vemulapalli M, Nutche J, Montrose DM, Sweeney JA, Pettegrew JW et al. Reduced N-acetyl-aspartate levels in schizophrenia patients with a younger onset age: a single-voxel 1H spectroscopy study. Schizophr Res 2007; 93: 23–32.
Tebartz van Elst L, Valerius G, Buchert M, Thiel T, Rusch N, Bubl E et al. Increased prefrontal and hippocampal glutamate concentration in schizophrenia: evidence from a magnetic resonance spectroscopy study. Biol Psychiatry 2005; 58: 724–730.
Theberge J, Al-Semaan Y, Williamson PC, Menon RS, Neufeld RWJ, Rajakumar N et al. Glutamate and glutamine in the anterior cingulate and thalamus of medicated patients with chronic schizophrenia and healthy comparison subjects measured with 4.0-T proton MRS. Am J Psychiatry 2003; 160: 2231–2233.
Olney JW, Farber NB . Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry 1995; 52: 998–1007.
van Erp TGM, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry 2015; 21: 547–553.
Roalf DR, Vandekar SN, Almasy L, Ruparel K, Satterthwaite TD, Elliott MA et al. Heritability of subcortical and limbic brain volume and shape in multiplex-multigenerational families with schizophrenia. Biol Psychiatry 2015; 77: 137–146.
Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry 2008; 64: 774–781.
Gur RE, Maany V, Mozley PD, Swanson C, Bilker W, Gur RC . Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am J Psychiatry 1998; 155: 1711–1717.
Moghaddam B, Javitt D . From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology 2012; 37: 4–15.
Liddle P, Friston K, Frith C, Hirsch S, Jones T, Frackowiak R . Patterns of cerebral blood flow in schizophrenia. Br J Psychiatry 1992; 160: 179–186.
Goghari VM, Sponheim SR, MacDonald AW . The functional neuroanatomy of symptom dimensions in schizophrenia: a qualitative and quantitative review of a persistent question. Neurosci Biobehav Rev 2010; 34: 468–486.
Ford JM, Roach BJ, Faustman WO, Mathalon DH . Synch before you speak: auditory hallucinations in schizophrenia. Am J Psychiatry 2007; 164: 458–466.
Stone J, Kotoula V, Dietrich C, De Simoni S, Krystal JH, Mehta MA . Perceptual distortions and delusional thinking following ketamine administration are related to increased pharmacological MRI signal changes in the parietal lobe. J Psychopharmacol 2015; 29: 1025–1028.
Stone JM, Dietrich C, Edden R, Mehta MA, De Simoni S, Reed LJ et al. Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol Psychiatry 2012; 17.
Acknowledgements
Thanks to the acquisition and recruitment team: Jacqueline Meeks, Jeff Valdez, Elliott Yodh, R. Sean Gallagher, Jason Blake, Prayosha Villa and Kevin Seelaus. This work was supported by National Institute of Mental Health R01MH099156 to BIT, K01MH102609 to DRR, P41 NIBIB EB015893 and R01 NINDS NS087516 to RR, and RC2 MH089983, P50 MH096891 & T32 MH019112 to REG. Additional support was provided by the Dowshen Program for Neuroscience at the University of Pennsylvania. The funding sources were not directly involved in study design, collection, data analysis or interpretation nor manuscript writing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website .
Supplementary information
Rights and permissions
About this article
Cite this article
Roalf, D., Nanga, R., Rupert, P. et al. Glutamate imaging (GluCEST) reveals lower brain GluCEST contrast in patients on the psychosis spectrum. Mol Psychiatry 22, 1298–1305 (2017). https://doi.org/10.1038/mp.2016.258
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2016.258
This article is cited by
-
Elevating the field for applying neuroimaging to individual patients in psychiatry
Translational Psychiatry (2024)
-
Neuroimaging genetics approaches to identify new biomarkers for the early diagnosis of autism spectrum disorder
Molecular Psychiatry (2023)
-
Characterizing the neurological phenotype of the hyperinsulinism hyperammonemia syndrome
Orphanet Journal of Rare Diseases (2022)
-
7T ultra-high-field neuroimaging for mental health: an emerging tool for precision psychiatry?
Translational Psychiatry (2022)
-
Genetics of glutamate and its receptors in autism spectrum disorder
Molecular Psychiatry (2022)