Abstract
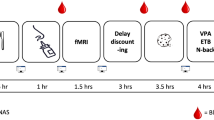
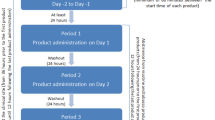
Many cigarette smokers express a desire to quit smoking, but ~85% of cessation attempts fail. In our attempt to delineate genetic modulators of smoking persistence, we have earlier shown that a locus within an ~250 kb haplotype block spanning the 5' untranslated region region of insulin-degrading enzyme is associated with serum cotinine levels; the study’s measure of smoking quantity. Based on our findings, and coupled with recent preclinical studies showing the importance of multiple neuropeptides in reinstatement of drug use, we formulated intranasal insulin to evaluate its efficacy during acute abstinence from smoking. Our original study was a crossover trial including 19 otherwise healthy smokers who abstained from smoking for 36 h. The morning following their second night of abstinence, in random order, study participants received intranasal insulin (60 IU) or placebo (8.7% sodium chloride). The goal of our second study was to replicate the craving findings from the original trial and expand this research by including additional stress-related measures. Thirty-seven study participants abstained from smoking overnight. The next day, they were administered either intranasal insulin (60 IU) or placebo, following which they participated in the Trier Social Stress Test Task. This was a parallel design study focusing on the standard stress subjective, hormonal and cardiovascular measures. We also evaluated any changes in circulating glucose, insulin and c-peptide (a marker of endogenous insulin). In the original study, intranasal insulin significantly reduced morning nicotine craving (b=3.65, P⩽0.05). Similarly, in the second study, intranasal insulin reduced nicotine cravings over time (b=0.065, P⩽0.05) and the effect lasted through the psychosocial stress period. Intranasal insulin also increased circulating cortisol levels (F=12.78, P⩽0.001). No changes in insulin or c-peptide were detected. A significant treatment × time interaction (P⩽0.05) was detected for glucose, but subjects remained well within the euglycemic range. Previous studies have shown that heightened nicotine cravings and blunted response to stress are independent and significant predictors of relapse to smoking. In our study, intranasal insulin normalized the subjective and hormonal response to stress. As such, intranasal insulin should further be studied in a larger clinical trial of smoking cessation. In support of this, we provide evidence that the treatment is safe and effective and, based on absence of peripheral insulin changes, conclude that the pharmacodynamic effect is centrally driven.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Tiffany ST, Wray JM . The clinical significance of drug craving. Ann NY Acad Sci 2012; 1248: 1–17.
Ferguson SG, Shiffman S . The relevance and treatment of cue-induced cravings in tobacco dependence. J Subst Abuse Treat 2009; 36: 235–243.
Brandon TH, Drobes DJ, Unrod M, Heckman BW, Oliver JA, Roetzheim RC et al. Varenicline effects on craving, cue reactivity, and smoking reward. Psychopharmacology (Berl) 2011; 218: 391–403.
Kwako LE, Spagnolo PA, Schwandt ML, Thorsell A, George DT, Momenan R et al. The corticotropin releasing hormone-1 (CRH1) receptor antagonist pexacerfont in alcohol dependence: a randomized controlled experimental medicine study. Neuropsychopharmacology 2015; 40: 1053–1063.
Al'Absi M, Hatsukami D, Davis GL . Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology 2005; 181: 107–117.
Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ . Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry 2011; 68: 942–952.
Hamidovic A, Goodloe RJ, Bergen AW, Benowitz NL, Styn MA, Kasberger JL et al. Gene-centric analysis of serum cotinine levels in African and European American populations. Neuropsychopharmacology 2012; 37: 968–974.
Schank JR, Ryabinin AE, Giardino WJ, Ciccocioppo R, Heilig M . Stress-related neuropeptides and addictive behaviors: beyond the usual suspects. Neuron 2012; 76: 192–208.
Mineur YS, Abizaid A, Rao Y, Salas R, DiLeone RJ, Gündisch D et al. Nicotine decreases food intake through activation of POMC neurons. Science 2011; 332: 1330–1332.
Seeley RJ, Woods SC . Monitoring of stored and available fuel by the CNS: implications for obesity. Nat Rev Neurosci 2003; 4: 901–909.
Exley R, Cragg SJ . Presynaptic nicotinic receptors: a dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. Br J Pharmacol 2008; 153 (Suppl 1): S283–S297.
Cachope R, Mateo Y, Mathur BN, Irving J, Wang HL, Morales M et al. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep 2012; 2: 33–41.
Stouffer MA, Woods CA, Patel JC, Lee CR, Witkovsky P, Bao L et al. Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nat Commun 2015; 6: 8543.
Labouèbe G, Liu S, Dias C, Zou H, Wong JC, Karunakaran S et al. Insulin induces long-term depression of ventral tegmental area dopamine neurons via endocannabinoids. Nat Neurosci 2013; 16: 300–308.
Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL . Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci 2002; 5: 514–516.
Middleton ET, Morice AH . Breath carbon monoxide as an indication of smoking habit. Chest 2000; 117: 758–763.
Stunkard AJ, Messick S . The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 1985; 29: 71–83.
Selzer ML . The Michigan alcoholism screening test: the quest for a new diagnostic instrument. Am J Psychiatry 1971; 127: 1653–1658.
McKee SA, Weinberger AH, Shi J, Tetrault J, Coppola S . Developing and validating a human laboratory model to screen medications for smoking cessation. Nicotine Tob Res 2012; 14: 1362–1371.
Cox LS, Tiffany ST, Christen AG . Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res 2001; 3: 7–16.
Watson D, Clark LA, Tellegen A . Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 1988; 54: 1063–1070.
Shiffman S, Hickcox M, Paty JA, Gnys M, Richards T, Kassel JD . Individual differences in the context of smoking lapse episodes. Addict Behav 1997; 22: 797–811.
NicotrolNS(R) [package insert]. Pfizer, New York, NY; January 2010; Available at: https://www.pfizer.com/files/products/uspi_nicotrol.pdf.
Claxton A, Baker LD, Hanson A, Trittschuh EH, Cholerton B, Morgan A et al. Long acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer's disease dementia. J Alzheimers Dis 2015; 45: 1269–1270.
Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol 2012; 69: 29–38.
Hamidovic A . Position on zinc delivery to olfactory nerves in intranasal insulin phase I-III clinical trials. Contemp Clin Trials 2015; 45 (Pt B): 277–280.
Lehrer S . Adverse side effects of intranasal detemir insulin in the SNIFF Trial. J Alzheimers Dis 2015; Available at: http://www.j-alz.com/content/adverse-side-effects-intranasal-detemir-insulin-sniff-trial.
Woods CA, Guttman ZR, Huang D, Kolaric RA, Rabinowitsch AI, Jones KT et al. Insulin receptor activation in the nucleus accumbens reflects nutritive value of a recently ingested meal. Physiol Behav 2016; 159: 52–63.
Figlewicz DP, Bennett JL, Aliakbari S, Zavosh A, Sipols AJ . Insulin acts at different CNS sites to decrease acute sucrose intake and sucrose self-administration in rats. Am J Physiol Regul Integr Comp Physiol 2008; 295: R388–R394.
Lee J, Finkelstein J, Choi JY, Witten IB . Linking cholinergic interneurons, synaptic plasticity, and behavior during the extinction of a cocaine-context association. Neuron 2016; 90: 1071–1085.
Chan O, Inouye K, Akirav E, Park E, Riddell MC, Vranic M et al. Insulin alone increases hypothalamo-pituitary-adrenal activity, and diabetes lowers peak stress responses. Endocrinology 2005; 146: 1382–1390.
Bohringer A, Schwabe L, Richter S, Schachinger H . Intranasal insulin attenuates the hypothalamic-pituitary-adrenal axis response to psychosocial stress. Psychoneuroendocrinology 2008; 33: 1394–1400.
Obici S, Zhang BB, Karkanias G, Rossetti L . Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 2002; 8: 1376–1382.
Kimura K, Tanida M, Nagata N, Inaba Y, Watanabe H, Nagashimada M et al. Central insulin action activates kupffer cells by suppressing hepatic vagal activation via the nicotinic alpha 7 acetylcholine receptor. Cell Rep 2016; 14: 2362–2374.
Ruigrok MJR, de Lange ECM . Emerging Insights for translational pharmacokinetic and pharmacokinetic-pharmacodynamic studies: towards prediction of nose-to-brain transport in humans. AAPS J 2015; 17: 493–505.
Illum L . Is nose-to-brain transport of drugs in man a reality? J Pharm Pharmacol 2004; 56: 3–17.
Dhuria SV, Hanson LR, Frey WH . Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Scie 2010; 99: 1654–1673.
Balin BJ, Broadwell RD, Salcman M, El-Kalliny M . Avenues for entry of peripherally administered protein to the central nervous system in mouse, rat, and squirrel monkey. J Comp Neurol 1986; 251: 260–280.
Salameh TS, Bullock KM, Hujoel IA, Wolden-Hanson T, Banks WA, Niehoff ML et al. Central nervous system delivery of intranasal insulin: mechanisms of uptake and effects on cognition. J Alzheimers Dis 2015; 47: 715–728.
Acknowledgements
This work was supported by NIH/NIDA (1R03DA036054 and 1R03DA03827), American Cancer Society Institutional Research Grant IRG-92-024, 8UL1TR000041 and P-50 AA022534.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Hamidovic, A., Khafaja, M., Brandon, V. et al. Reduction of smoking urges with intranasal insulin: a randomized, crossover, placebo-controlled clinical trial. Mol Psychiatry 22, 1413–1421 (2017). https://doi.org/10.1038/mp.2016.234
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2016.234
This article is cited by
-
G-CuP: the effect of a forced oral glucose intake on alcohol craving and mesolimbic cue reactivity in alcohol dependence—study protocol of a randomized, double-blind, placebo-controlled crossover study
Trials (2022)
-
Intranasal calcitonin gene-related peptide administration impairs fear memory retention in mice through the PKD/p-HDAC5/Npas4 pathway
Scientific Reports (2022)
-
Metabolic and Addiction Indices in Patients on Opioid Agonist Medication-Assisted Treatment: A Comparison of Buprenorphine and Methadone
Scientific Reports (2020)
-
Intranasal Insulin: a Treatment Strategy for Addiction
Neurotherapeutics (2020)