Abstract
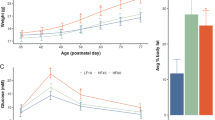
Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder of unknown etiology, but very likely resulting from both genetic and environmental factors. There is good evidence for immune system dysregulation in individuals with ASD. However, the contribution of insults such as dietary factors that can also activate the immune system have not been explored in the context of ASD. In this paper, we show that the dietary glycemic index has a significant impact on the ASD phenotype. By using BTBR mice, an inbred strain that displays behavioral traits that reflect the diagnostic symptoms of human ASD, we found that the diet modulates plasma metabolites, neuroinflammation and brain markers of neurogenesis in a manner that is highly reflective of ASD in humans. Overall, the manuscript supports the idea that ASD results from gene–environment interactions and that in the presence of a genetic predisposition to ASD, diet can make a large difference in the expression of the condition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Herbert MR . Contributions of the environment and environmentally vulnerable physiology to autism spectrum disorders. Curr Opin Neurol 2010; 23: 103–110.
Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T et al. Genetic heritability and shared environmental factors among twin pairs with autim. Arch Gen Psychiatry 2011; 68: 1095–1102.
Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA . Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol 2005; 57: 67–81.
Patterson PH . Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res 2009; 204: 313–321.
Michel M, Schmidt MJ, Mirnics K . Immune system gene dysregulation in autism and schizophrenia. Dev Neurobiol 2012; 72: 1277–1287.
Meyer U, Feldon J, Dammann O . Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation. Pediatr Res 2011; 69: 26R–33R.
Donath MY, Shoelson SE . Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011; 11: 98–107.
Lumeng CN, Saltiel AR . Inflammatory links between obesity and metabolic disease. J Clin Invest 2011; 121: 2111–2117.
Lyall K, Pauls DL, Santangelo S, Spiegelman D, Ascherio A . Maternal early life factors associated with hormone levels and the risk of having a child with an autism spectrum disorder in the Nurses Health Study II. J Autism Dev Disord 2011; 41: 618–627.
Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL et al. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics 2012; 129: e1121.
Pepys MB, Hirschfield GM . C-reactive protein: a critical update. J Clin Invest 2003; 111: 1805–1812.
Lin J, Zhang M, Song F, Qin J, Wang R, Yao P et al. Association between C-reactive protein and pre-diabetic status in a Chinese Han clinical population. Diabetes Metab Res Rev 2009; 25: 219–223.
Wang X, Bao W, Liu J, Ouyang YY, Wang D, Rong S et al. Inflammatory markers and risk of type 2 diabetes. Diabetes Care 2013; 36: 1166–1175.
Choi J, Joseph L, Pilote L . Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev 2013; 14: 232–244.
Brown AS, Sourander A, Hinkka-Yli-Salomaki S, McKeague IW, Sundvall J, Surcel H-M . Elevated maternal C-reactive protein and autism in a national birth cohort. Mol Psychiatry 2013; 19: 259–264.
Galland L . Diet and inflammation. Nutr Clin Pract 2010; 25: 634–640.
Neuhouser ML, Schwarz Y, Wang CX, Breymeyer K, Coronado G, Wang C-Y et al. A low glycemic load diet reduces serum C-reactive protein and modestly increases adiponectin in overweight and obese adults. J Nutr 2012; 142: 369–374.
Livesey G, Taylor R, Hulshof T, Howlett J . Glycemic response and health—a systematic review and meta-analysis: relations between dietary glycemic properties and health outcomes. Am J Clin Nutr 2008; 87: 258S–268S.
de Carvalho Vidigal F, Guedes Cocate P, Goncalves Periera L, de Cassia Goncalves Alfenas R . The role of hyperglycemia in the induction of oxidative stress and inflammatory process. Nutr Hosp 2012; 27: 1391–1398.
Uchiki T, Weikel KA, Jiao W, Shang F, Caceres A, Pawlak D et al. Glycation-altered proteolysis as a pathobiologic mechanism that links dietary glycemic index, aging, and age-related disease (in non-diabetics). Aging Cell 2012; 11: 1–13.
Livadas S, Dastamani A, Dikaiakos A, Mantzou A, Chrousos GP . Diet with low glycemic index, low glycemic load dessert consumption decreases serum advanced glycation end products concentrations in both overweight/obese children and adults undergoing nutritional intervention. Endocr Rev 2013; 34: MON-349.
Vlassara H, Striker GE . AGE restriction in diabetes mellitus: a paradigm shift. Nat Rev Endocrinol 2011; 7: 526–539.
Fleming TH, Humpert PM, Nawroth PP, Bierhaus A . Reactive metabolites and AGE/RAGE-mediated cellular dysfunction affect the aging process-a mini-review. Gerontol 2011; 57: 435–443.
Desai KM, Chang T, Wang H, Banigesh A, Dhar A, Liu J et al. Oxidative stress and aging: is methylglyoxal the hidden enemy? Can J Physiol Pharmacol 2010; 88: 273–284.
Maher P . Methylglyoxal, advanced glycation end products and autism: is there a connection? Med Hypotheses 2012; 78: 548–552.
Silverman JL, Yang MH, Lord C, Crawley JN . Behavioral phenotyping assays for mouse models of autism. Nat Rev Neurosci 2010; 11: 490–502.
Meyza KZ, Defensor EB, Jensen AL, Corley MJ, Pearson BL, Pobbe RLH et al. The BTBR T+tf/J mouse model for autism spectrum disorders-in search of biomarkers. Behav Brain Res 2013; 251: 25–34.
Weikel KA, FitzGerald P, Shang F, Caceres MA, Bian Q, Hanada JT et al. Natural history of age-related retinal lesions that precede AMD in mice fed high or low glycemic index diets. Invest Ophthalmol Vis Sci 2012; 53: 622–632.
Crawley JN . Translational animal models of autism and neurodevelopmental disorders. Dialogues Clin Neurosci 2012; 14: 293–305.
Sanderson DJ, Good MA, Skelton K, Sprengel R, Seeburg PH, Rawlins JN et al. Enhanced long-term and impaired short-term spatial memory in GluA1 AMPA receptor subunit knockout mice: evidence for dual-process memory model. Learn Mem 2009; 16: 379–386.
McCullagh EA, Featherstone DE . Behavioral characterization of system xc- mutant mice. Behav Brain Res 2014; 265: 1–11.
Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH . Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun 2012; 26: 607–616.
Xia J, Mandal R, Sinelnikov IV, Broadhurst D, Wishart DS . MetaboAnalyst 2.0—a comprehensive server for metabolomic data analysis. Nucleic Acids Res 2012; 40: W127–W133.
Garay PA, Hsiao EY, Patterson PH, McAllister AK . Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav Immun 2013; 31: 54–68.
Stephenson DT, O'Neil SM, Narayan S, Tiwari A, Arnold E, Samaroo HD et al. Histopathologic characterization of the BTBR mouse model of autistic-like behavior reveals selective changes in neurodevelopmental proteins and adult hippocampal neurogenesis. Mol Autism 2011; 2: 7.
Huguet G, Ey E, Bourgeron T . The genetic landscape of autism spectrum disorders. Annu Rev Genomics Hum Genet 2013; 14: 191–213.
Harry GJ . Microglia during development and aging. Pharmacol Ther 2013; 139: 313–326.
Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci 2014; 17: 400–406.
Kaminska B, Gozdz A, Zawadzka M, Ellert-Miklaszewska A, Lipko M . MAPK signal transduction underlying brain inflammation and gliosis as therapeutic target. Anat Rec 2009; 292: 1902–1913.
Edmonson C, Ziats MN, Rennert OM . Altered glial marker expression in autistic post-mortem prefrontal cortex and cerebellum. Mol Autism 2014; 5: 3.
Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC et al. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA 2009; 106: 3698–3703.
Pardo CA, Eberhart CG . The neurobiology of autism. Brain Pathol 2007; 17: 434–447.
Wegiel J, Kuchna I, Nowicki K, Imaki H, Wegiel J, Marchi E et al. The neuropathology of autism: defects of neurogenesis and neuronal migration, and dysplastic changes. Acta Neuropathol 2010; 119: 755–770.
Stoner R, Chow ML, Boyle MP, Sunkin SM, Mouton PR, Roy S et al. Patches of disorganization in the neocortex of children with autism. N Engl J Med 2014; 370: 1209–1219.
Groen WB, Buitelaar JK, van der Gaag RJ, Zwiers MP . Pervasive microstructural abnormalities in autism: a DTI study. J Psychiatry Neurosci 2011; 36: 32–40.
Vlassara H, Cai W, Crandall J, Goldberg T, Oberstein R, Dardaine V et al. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci USA 2002; 99: 15596–15601.
Stirban A, Negrean M, Gotting C, Uribarri J, Gawlowski T, Stratmann B et al. Dietary advance glycation endproducts and oxidative stress. Ann NY Acad Sci 2008; 1126: 276–279.
Rutkowsky JM, Knotts TA, Ono-Moore KD, McCoin CS, Huang S, Schneider D et al. Acylcarnitines activate proinflammatory signaling pathways. Am J Physiol Endocrinol Metab 2014; 306: E1378–E1387.
Bakala H, Ladouce R, Baraibar MA, Friguet B . Differential expression and glycative damage affect specific mitochondrial proteins with aging in rat liver. Biochim Biophys Acta 2013; 1832: 2057–2067.
Kohman RA, Rhodes JS . Neurogenesis, inflammation and behavior. Brain Behav Immun 2013; 27: 22–32.
Liu Y-H, Lai W-S, Tsay H-J, Wang T-W, Yu J-Y . Effects of maternal immune activation on adult neurogenesis in the subventricular zone-olfactory bulb pathway and olfactory discrimination. Schizophr Res 2013; 151: 1–11.
Gleeson JG, Lin PT, Flanagan LA, Walsh CA . Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron 1999; 23: 257–271.
Dinarello CA, Novick D, Kim S, Kaplanski G . Interleukin-18 and IL-18 binding protein. Front Immunol 2013; 4: 289.
Krumbholz M, Theil D, Steinmeyer F, Cepok S, Hemmer B, Hofbauer M et al. Ccl19 is constitutively expressed in the CNS, up-regulated in neuroinflammation, active and also inactive multiple sclerosis lesions. J Neuroimmunol 2007; 190: 72–79.
Kaul D, Habbel P, Derkow K, Kruger C, Franzoni E, Wulczyn FG et al. Expression of Toll-like receptors in the developing brain. PLoS One 2012; 7: e37767.
Mocco J, Mack WJ, Ducruet AF, Sosunov SA, Sughrue ME, Hassid BG et al. Complement component C3 mediates inflammatory injury following focal cerebral ischemia. Circ Res 2006; 99: 209–271.
Tremblay M-E, Stevens BR, Sierra A, Wake H, Bessis A, Nimmerjahn A . The role of microglia in the healthy brain. J Neurosci 2011; 31: 16064–16069.
Momeni N, Bergquist J, Brudin L, Behnia F, Sivberg B, Joghataei MT et al. A novel blood-based biomarker for detection of autism spectrum disorders. Transl Psychiatry 2012; 2: e91.
Chen Y, Guillemin GJ . Kynurenine pathway metabolites in humans: disease and healthy states. Int J Tryptophan Res 2009; 2: 1–19.
Frye RE, Melnyk S, MacFabe DF . Unique acyl-carnitine profiles are potential biomarkers for acquired mitochondrial disease in autism spectrum disorder. Transl Psychiatry 2013; 3: e220.
Joels M, Sarabdjitsingh A, Karst H . Unraveling the time domains of corticosteroid influences on brain activity: rapid, slow and chronic modes. Pharmacol Rev 2012; 64: 901–938.
Naviaux JC, Schuchbauer MA, Li K, Wang L, Risbrough VB, Powell SB et al. Reversal of autism-like behaviors and metabolism in adult mice with single-dose antipurinergic therapy. Transl Psychiatry 2014; 4: e400.
Kuo S-M . The interplay between fiber and the intestinal microbiome in the inflammatory response. Adv Nutr 2013; 4: 16–28.
Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013; 155: 1451–1463.
Geier DA, Kern JK, Garver CR, Adams JB, Audhya T, Geier MR . A prospective study of transsulfuration biomarkers in autistic disorders. Neurochem Res 2009; 34: 386–393.
Frustaci A, Neri M, Cesario A, Adams JB, Domenici E, Dalla Bernardina B et al. Oxidative stress-related biomarkers in autism: systematic review and meta-analyses. Free Radic Biol Med 2012; 52: 2128–2141.
Naushad SM, Jain JM, Prasad CK, Naik U, Akella RR . Autistic children exhibit distinct plasma amino acid profile. Indian J Biochem Biophys 2013; 50: 474–478.
Ming X, Stein TP, Barnes V, Rhodes N, Guo L . Metabolic perturbance in autism spectrum disorders: a metabolomics study. J Proteome Res 2012; 11: 5856–5862.
James SJ . Autism and folate-dependent one-carbon metabolism: serendipity and critical branch-point decisions in science. Glob Adv Health Med 2013; 2: 48–51.
Mbadiwe T, Millis RM . Epigenetics and autism. Autism Res Treat 2013; 2013: 826156.
Srinivasan P . A review of dietary interventions in autism. Ann Clin Psychiatry 2009; 21: 237–247.
Marti LF . Effectiveness of nutritional interventions on the functioning of children with ADHD and/or ASD. Bol Asoc Med PR 2010; 102: 31–42.
Ruskin DN, Svedova J, Cote JL, Sandau U, Rho JM, Kawamura M et al. Ketogenic diet improves core symptoms of autism in BTBR mice. PLoS One 2013; 8: e65021.
Acknowledgements
This work was supported by the Fritz B. Burns Foundation. We thank Dr Christine Pelkman from Ingredion for supplying the starches for the diets.
Author Contributions
PM designed the study. AC, CF, RD, MG-S and PM performed the experiments and analyzed the data. AC, CF and PM wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Currais, A., Farrokhi, C., Dargusch, R. et al. Dietary glycemic index modulates the behavioral and biochemical abnormalities associated with autism spectrum disorder. Mol Psychiatry 21, 426–436 (2016). https://doi.org/10.1038/mp.2015.64
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2015.64
This article is cited by
-
Low glycemic index diet restrains epileptogenesis in a gender-specific fashion
Cellular and Molecular Life Sciences (2023)
-
Neonatal curcumin treatment restores hippocampal neurogenesis and improves autism-related behaviors in a mouse model of autism
Psychopharmacology (2020)
-
Methylglyoxal-Induced Protection Response and Toxicity: Role of Glutathione Reductase and Thioredoxin Systems
Neurotoxicity Research (2017)