Abstract
Brain neuroplasticity is increasingly considered to be an important component of both the pathology and treatment of depressive spectrum disorders. Recent studies shed light on the relevance of hippocampal cell genesis and cortico-limbic dendritic plasticity for the development and remission from depressive-like behavior. However, the neurobiological significance of neuroplastic phenomena in this context is still controversial. Here we summarize recent developments in this topic and propose an integrative interpretation of data gathered so far.
Similar content being viewed by others
Introduction
The potential to respond to environmental stimuli through dynamic rearrangements of synapto-dendritic networks, as well as by regulating the generation of new neuronal and glial cells, renders the brain highly mutable. These phenomena, collectively known as neuroplasticity, are critical to promote neuronal adaptations; its failure is now increasingly considered to be a major component in many neuropsychiatric conditions. Among these, depressive spectrum disorders are a paradigmatic example of the importance of neuroplastic alterations in the adult brain. Recent studies provide a comprehensive picture of the effects of stress, a major trigger factor in depression, in the (de)regulation of neuroplasticity;1, 2, 3, 4 the latter is, in turn, related to the emergence of physiological and behavioral alterations comprised in the symptomatic profile of depressive disorders. Although these molecular and physiological mechanisms regulating neuroplastic processes are relevant for the onset of depressive symptoms, they have also been implicated in the action of antidepressants (ADs). So far, and although there is still much to be elucidated, it is becoming evident that the triad stress-neuroplasticity-depression constitutes fertile ground for new findings.
New cells and dendrites: importance for the treatment and remission from depression
Although different forms of neuroplasticity are affected in depression, a debate endures concerning the exact neurobiological significance of postnatal hippocampal cell genesis, both for the development of depressive pathology and for the therapeutic action of ADs. From the bulk of evidence gathered so far, it is increasingly appreciated that alterations in cell genesis are involved in the pathology and treatment of depression; however, there are several conflicting reports regarding its relevance. Three factors are likely to contribute to these controversies. First, there is a ‘necessary’ difficulty to approach this question in humans suffering from depression; postmortem studies in humans and animal models of depression have, nevertheless, provided important insights. Second, it seems to exist a major prevalence of studies focusing on the functional implications of neurogenesis, in disregard of gliogenesis, a parallel cell-genesis process likely to be of relevance in this context. Lastly, because these events are highly dynamic, the adoption of different experimental models and time frames when analyzing the participation of cell genesis in the pathology and treatment of depression is critical to have a complete perspective of the topic. On account of these experimental dissimilarities, an integrative, and careful, interpretation of data published in the last years is required when attempting to put the pieces of the puzzle together.
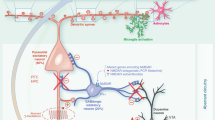
Suppression of hippocampal cell proliferation in naive animals through irradiation, pharmacological approaches or through the use of transgenic models of cytogenesis ablation has been shown to be associated with the development of deficits in different behavioral dimensions commonly affected in depression.2, 5, 6 Strikingly, most of the studies in which analyses were performed shortly after cytogenesis ablation did not reveal significant deficits in most behavioral domains normally assessed in the characterization of animal models of depression (Figure 1).7, 8 However, recent reports in which abrogation of cytogenesis is maintained for long periods (over 4 weeks)6 or in which the behavioral analysis was conducted only 4 weeks after the cessation of cytogenesis suppression,4 reported multidimensional behavioral deficits that emerged only weeks after the antiproliferative insult. Importantly, the specific late manifestation of depressive-like behavior and cognitive disabilities in animals in which cytogenesis had been suppressed illustrates how manipulating lengthy neuroplastic phenomena is associated with the non-immediate development of behavioral impairments, which are only fully manifested once newborn cells are expected to be incorporated in local neuroglial circuits. This view has been recently supported by the demonstration that the specific inhibition of 4-week old new hippocampal neurons causes deficits in memory retrieval in mice; remarkably, inhibiting the activity of either younger or less-plastic older neurons does not produce effects in this cognitive domain.9
The participation of dendritic plasticity and hippocampal cell genesis in the development and treatment of depression. (a) In animal models of depression, chronic exposure to stress, one major trigger factor of depression, leads to the dendritic atrophy of hippocampal and prefrontal cortex neurons and compromises the generation of new cells. These neuroplastic impairments are associated with the development of multidimensional deficits manifested in behavioral dimensions such as mood, anxiety behavior and cognitive performance. Treatment with typical SSRIs allows for the fast reestablishment of dendritic neuronal architecture, which is associated with the fast remission from depression-like behavior. The slow generation of new neuronal and glial cells, potentiated by antidepressant (AD) treatment will be fundamental to maintain this remission and to promote a sustained long-term recovery from depression. (b) Suppression of hippocampal cell genesis, using X-ray irradiation, the cytostatic agent methylazoxymethanol or transgenic animal models of cytogenesis ablation, does not seem to have an impact on the mood-improving effects of ADs, but rather to preclude AD’s efficacy in reversing anxiety behavior and impairments in some cognitive modalities (blue line); other cognitive functions remain unaffected (dashed blue line). (c) Ablation of cytogenesis in naive animals, while maintaining dendritic morphology intact, leads to the short-term manifestation of hyperanxiety behavior; deficits in mood and cognitive modalities are only evident later on, when newborn cells are expected to be fully matured and incorporated in the local neurocircuitry. SSRIs, selective serotonin reuptake inhibitors.
An exception must be made in respect to anxiety behavior, because disruption of hippocampal cytogenesis is associated to the immediate development of heightened anxiety, which is commonly comorbid in depressed patients. In fact, it has been demonstrated that immature newborn neurons display a major role in anxiety behavior control.10, 11, 12
In contrast with the slow reconfiguration of neuroglial networks promoted by the addition of new cells in the adult brain, are the rapid synaptic and dendritic morphological changes. These underlie the short-term impacts on distinct emotional and cognitive processes observed in the onset of depressive symptoms. Indeed, defects on neuronal cytoarchitecture have been documented in postmortem analysis of brain tissue from depressive patients, which seem to be ameliorated by chronic AD treatment.13, 14 These structural defects include cortico-limbic dendritic atrophy in depressed subjects,15 accompanied by abnormalities in glial cell structure. Importantly, in animal models of depression, these changes are associated with hallmarks of depressive behavior, such as anhedonic behavior, behavioral despair and cognitive disabilities.2
Besides this common participation of slow cytogenesis processes and rapid dendritic rearrangements in the pathology of depressive spectrum disorders, these two neuroplastic phenomena, with very different temporal dynamics, constitute the substrate by which ADs (partially) exert their therapeutic actions. Thus, the reestablishment of normal neuroglial networks seems to be achieved in a biphasic manner (Figure 1): in a short-term context, AD actions rely on rapid modulatory effects upon genes involved in the restructuring of the synaptic network; later on, the generation of new fully matured neuronal and glial cells will have an impact on the long-term remission from emotional and cognitive disabilities manifested during a depressive episode. In fact, despite triggering an immediate pro-proliferative response, this early effect corresponds only to the onset of a slow neuroadaptation whose neurobiological importance can only be fully appreciated later on, once new cells attain complete maturation and functionality, and are integrated in the local neurocircuitry. Taken together, and because mammalian neurogenesis is described to take 4–6 weeks, the overall outcome of AD’s therapeutic action upon neuroplasticity may only be entirely manifested after this period. Remarkably, this period correlates with the time latency that typically prescribed ADs take to fully manifest their action in depressive patients.
Although the neuroplastic alterations occurring during the onset, treatment and remission from depression are being increasingly characterized, comprehension of the processes (namely genetic and epigenetic programs) that orchestrate these alterations is still limited. Future research focusing on these processes should also be extended to the still underexplored glioplastic component of this disorder. Furthermore, local neuroplastic adaptations are likely to occur in articulation with systemic neuroendocrine and immunological alterations, which are still to be integrated in the complex puzzle of mechanisms implicated in depression.
References
Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C et al. Antidepressants recruit new neurons to improve stress response regulation. Mol Psychiatry 2011; 16: 1177–1188.
Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA et al. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry 2009; 14: 764–773, 739.
Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL et al. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry 2010; 15: 501–511.
Mateus-Pinheiro A, Pinto L, Bessa JM, Morais M, Alves ND, Monteiro S et al. Sustained remission from depressive-like behavior depends on hippocampal neurogenesis. Transl Psychiatry 2013; 3: e210.
Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003; 301: 805–809.
Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA . Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 2011; 476: 458–461.
Jayatissa MN, Henningsen K, West MJ, Wiborg O . Decreased cell proliferation in the dentate gyrus does not associate with development of anhedonic-like symptoms in rats. Brain Res 2009; 1290: 133–141.
David DJ, Samuels BA, Rainer Q, Wang J-W, Marsteller D, Mendez I et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 2009; 62: 479–493.
Gu Y, Arruda-Carvalho M, Wang J, Janoschka SR, Josselyn SA, Frankland PW et al. Optical controlling reveals time-dependent roles for adult-born dentate granule cells. Nat Neurosci 2012; 15: 1700–1706.
Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV et al. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry 2009; 14: 959–967.
Kirby ED, Friedman AR, Covarrubias D, Ying C, Sun WG, Goosens KA et al. Basolateral amygdala regulation of adult hippocampal neurogenesis and fear-related activation of newborn neurons. Mol Psychiatry 2012; 17: 527–536.
Sah A, Schmuckermair C, Sartori SB, Gaburro S, Kandasamy M, Irschick R et al. Anxiety- rather than depression-like behavior is associated with adult neurogenesis in a female mouse model of higher trait anxiety- and comorbid depression-like behavior. Transl Psychiatry 2012; 2: e171.
Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY et al. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry 2004; 56: 640–650.
Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry 1999; 45: 1085–1098.
Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP . Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex 2002; 12: 386–394.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
PowerPoint slides
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Mateus-Pinheiro, A., Patrício, P., Bessa, J. et al. Cell genesis and dendritic plasticity: a neuroplastic pas de deux in the onset and remission from depression. Mol Psychiatry 18, 748–750 (2013). https://doi.org/10.1038/mp.2013.56
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2013.56
Keywords
This article is cited by
-
Hippocampal cytogenesis abrogation impairs inter-regional communication between the hippocampus and prefrontal cortex and promotes the time-dependent manifestation of emotional and cognitive deficits
Molecular Psychiatry (2021)
-
Inducing a long-term potentiation in the dentate gyrus is sufficient to produce rapid antidepressant-like effects
Molecular Psychiatry (2018)
-
Chronic stress targets adult neurogenesis preferentially in the suprapyramidal blade of the rat dorsal dentate gyrus
Brain Structure and Function (2018)
-
Adult hippocampal neuroplasticity triggers susceptibility to recurrent depression
Translational Psychiatry (2017)
-
AP2γ controls adult hippocampal neurogenesis and modulates cognitive, but not anxiety or depressive-like behavior
Molecular Psychiatry (2017)