Abstract
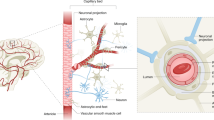
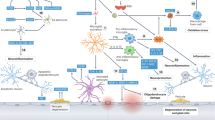
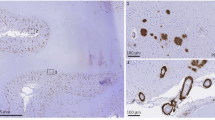
Alzheimer’s disease (AD) and vascular dementia are the major causes of cognitive disorders worldwide. They are characterized by cognitive impairments along with neuropsychiatric symptoms, and that their pathogeneses show overlapping multifactorial mechanisms. Although AD has long been considered the most common cause of dementia, individuals afflicted with AD commonly exhibit cerebral vascular abnormalities. The concept of mixed dementia has emerged to more clearly identify patients with neurodegenerative phenomena exhibiting both AD and cerebral vascular pathologies—vascular damage along with β-amyloid (Aβ)-associated neurotoxicity and τ-hyperphosphorylation. Cognitive impairment has long been commonly explained through a ‘neuro-centric’ perspective, but emerging evidence has shed light over the important roles that neurovascular unit dysfunction could have in neuronal death. Moreover, accumulating data have been demonstrating astrocytes being the essential cell type in maintaining proper central nervous system functioning. In relation to dementia, the roles of astrocytes in Aβ deposition and clearance are unclear. This article emphasizes the multiple events triggered by ischemia and the cytotoxicity exerted by Aβ either alone or in association with endothelin-1 and receptor for advanced glycation end products, thereby leading to neurodegeneration in an ‘astroglio-centric’ perspective.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Qiu C, De Ronchi D, Fratiglioni L . The epidemiology of the dementias: an update. Curr Opin Psychiatry 2007; 20: 380–385.
Galimberti D, Scarpini E . Progress in Alzheimer's disease. J Neurol 2012; 259: 201–211.
Brun A, Englund E . Regional pattern of degeneration in Alzheimer's disease: neuronal loss and histopathological grading. Histopathology 1981; 5: 549–564.
Brun A, Liu X, Erikson C . Synapse loss and gliosis in the molecular layer of the cerebral cortex in Alzheimer's disease and in frontal lobe degeneration. Neurodegeneration 1995; 4: 171–177.
Glenner GG, Wong CW . Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 1984; 120: 885–890.
Kosik KS, Joachim CL, Selkoe DJ . Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci USA 1986; 83: 4044–4048.
Enciu AM, Constantinescu SN, Popescu LM, Muresanu DF, Popescu BO . Neurobiology of vascular dementia. J Aging Res 2011; 2011: 401604.
Wallin A, Milos V, Sjogren M, Pantoni L, Erkinjuntti T . Classification and subtypes of vascular dementia. Int Psychogeriatr 2003; 15 ((Suppl 1)): 27–37.
Erkinjuntti T . Vascular cognitive deterioration and stroke. Cerebrovasc Dis 2007; 24 ((Suppl 1)): 189–194.
Lee AY . Vascular dementia. Chonnam Med J 2011; 47: 66–71.
Zlokovic BV . Vascular disorder in Alzheimer's disease: role in pathogenesis of dementia and therapeutic targets. Adv Drug Deliv Rev 2002; 54: 1553–1559.
Li J, Wang YJ, Zhang M, Fang CQ, Zhou HD . Cerebral ischemia aggravates cognitive impairment in a rat model of Alzheimer's disease. Life Sci 2011; 89: 86–92.
Langa KM, Foster NL, Larson EB . Mixed dementia: emerging concepts and therapeutic implications. JAMA 2004; 292: 2901–2908.
Zekry D, Hauw JJ, Gold G . Mixed dementia: epidemiology, diagnosis, and treatment. J Am Geriatr Soc 2002; 50: 1431–1438.
Schneider JA, Arvanitakis Z, Bang W, Bennett DA . Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007; 69: 2197–2204.
Sheng B, Cheng LF, Law CB, Li HL, Yeung KM, Lau KK et al. Coexisting cerebral infarction in Alzheimer's disease is associated with fast dementia progression: applying the National Institute for Neurological Disorders and Stroke/Association Internationale pour la Recherche et l'Enseignement en Neurosciences Neuroimaging Criteria in Alzheimer's Disease with Concomitant Cerebral Infarction. J Am Geriatr Soc 2007; 55: 918–922.
Zekry D, Gold G . Management of mixed dementia. Drugs Aging 2010; 27: 715–728.
Fillenbaum GG, van Belle G, Morris JC, Mohs RC, Mirra SS, Davis PC et al. Consortium to Establish a Registry for Alzheimer's Disease (CERAD): the first twenty years. Alzheimers Dement 2008; 4: 96–109.
Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R . Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer's Disease Diagnostic and Treatment Centers. Neurology 1992; 42: 473–480.
Jablonski M, Maciejewski R, Januszewski S, Ulamek M, Pluta R . One year follow up in ischemic brain injury and the role of Alzheimer factors. Physiol Res 2011; 60 ((Suppl 1)): S113–S119.
Garcia-Alloza M, Gregory J, Kuchibhotla KV, Fine S, Wei Y, Ayata C et al. Cerebrovascular lesions induce transient beta-amyloid deposition. Brain 2011; 134: 3697–3707.
Bell RD, Zlokovic BV . Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer's disease. Acta Neuropathol 2009; 118: 103–113.
Iadecola C . The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol 2010; 120: 287–296.
Yang GY, Betz AL . Reperfusion-induced injury to the blood-brain barrier after middle cerebral artery occlusion in rats. Stroke 1994; 25: 1658–1664.
Zhang X, Zhou K, Wang R, Cui J, Lipton SA, Liao FF et al. Hypoxia-inducible factor 1alpha (HIF-1alpha)-mediated hypoxia increases BACE1 expression and beta-amyloid generation. J Biol Chem 2007; 282: 10873–10880.
Zacchigna S, Lambrechts D, Carmeliet P . Neurovascular signalling defects in neurodegeneration. Nat Rev Neurosci 2008; 9: 169–181.
Schuff N, Matsumoto S, Kmiecik J, Studholme C, Du A, Ezekiel F et al. Cerebral blood flow in ischemic vascular dementia and Alzheimer's disease, measured by arterial spin-labeling magnetic resonance imaging. Alzheimers Dement 2009; 5: 454–462.
Cai Z, Zhao B, Ratka A . Oxidative stress and beta-amyloid protein in Alzheimer's disease. Neuromolecular Med 2011; 13: 223–250.
Verkhratsky A, Rodriguez JJ, Parpura V . Astroglia in neurological diseases. Future Neurol 2013; 8: 149–158.
Bushong EA, Martone ME, Jones YZ, Ellisman MH . Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 2002; 22: 183–192.
Ogata K, Kosaka T . Structural and quantitative analysis of astrocytes in the mouse hippocampus. Neuroscience 2002; 113: 221–233.
Sofroniew MV, Vinters HV . Astrocytes: biology and pathology. Acta Neuropathol 2010; 119: 7–35.
Miller RH, Raff MC . Fibrous and protoplasmic astrocytes are biochemically and developmentally distinct. J Neurosci 1984; 4: 585–592.
Emsley JG, Macklis JD . Astroglial heterogeneity closely reflects the neuronal-defined anatomy of the adult murine CNS. Neuron Glia Biol 2006; 2: 175–186.
Clarke LE, Barres BA . Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci 2013; 14: 311–321.
Oberheim NA, Goldman SA, Nedergaard M . Heterogeneity of astrocytic form and function. Methods Mol Biol 2012; 814: 23–45.
Perea G, Navarrete M, Araque A . Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 2009; 32: 421–431.
Takano T, Tian GF, Peng W, Lou N, Libionka w, Han X et al. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci 2006; 9: 260–267.
Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW et al. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci 2006; 9: 1397–1403.
Santello M, Volterra A . Synaptic modulation by astrocytes via Ca2+-dependent glutamate release. Neuroscience 2009; 158: 253–259.
Anderson CM, Swanson RA . Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia 2000; 32: 1–14.
Abbott NJ . Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat 2002; 200: 629–638.
Strohschein S, Hüttmann K, Gabriel S, Binder DK, Heinemann U, Stainhäuser C . Impact of aquaporin-4 channels on K+ buffering and gap junction coupling in the hippocampus. Glia 2011; 59: 973–980.
Malhotra SK, Svensson M, Aldskogius H, Bhatnagar R, Das GD, Shnitka TK . Diversity among reactive astrocytes: proximal reactive astrocytes in lacerated spinal cord preferentially react with monoclonal antibody J1-31. Brain Res Bull 1993; 30: 395–404.
Pekny M, Pekna M . Astrocyte intermediate filaments in CNS pathologies and regeneration. J Pathol 2004; 204: 428–437.
Wilhelmsson U, Bushong EA, Price DL, Smarr BL, Phung V, Terada M et al. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci USA 2006; 103: 17513–17518.
Oberheim NA, Tian GF, Han X, Peng W, Takano T, Ransom B et al. Loss of astrocytic domain organization in the epileptic brain. J Neurosci 2008; 28: 3264–3276.
Wanner IB, Anderson MA, Song B, Levine J, Fernandez A, Gray-Thompson Z et al. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci 2013; 33: 12870–12886.
Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN et al. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron 1999; 23: 297–308.
Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV . Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 2004; 24: 2143–2155.
Myer DJ, Gurkoff GG, Lee SM, Hovda DA, Sofroniew MV . Essential protective roles of reactive astrocytes in traumatic brain injury. Brain 2006; 129: 2761–2772.
Zhang D, Hu X, Qian L, O'Callaghan JP, Hong JS . Astrogliosis in CNS pathologies: is there a role for microglia? Mol Neurobiol 2010; 41: 232–241.
Funato H, Yoshimura M, Yamazaki T, Saido TC, Ito Y, Yokofujita J et al. Astrocytes containing amyloid beta-protein (Abeta)-positive granules are associated with Abeta40-positive diffuse plaques in the aged human brain. Am J Pathol 1998; 152: 983–992.
Chen Y, Vartiainen NE, Ying W, Chan PH, Koistinaho J, Swanson RA . Astrocytes protect neurons from nitric oxide toxicity by a glutathione-dependent mechanism. J Neurochem 2001; 77: 1601–1610.
Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG et al. Genomic analysis of reactive astrogliosis. J Neurosci 2012; 32: 6391–6410.
Hamby ME, Coppola G, Ao Y, Geschwind DH, Khakh BS, Sofroniew MW . Inflammatory mediators alter the astrocyte transcriptome and calcium signaling elicited by multiple G-protein-coupled receptors. J Neurosci 2012; 32: 14489–14510.
Koistinaho M, Koistinaho J . Interactions between Alzheimer's disease and cerebral ischemia—focus on inflammation. Brain Res Brain Res Rev 2005; 48: 240–250.
Pluta R, Ulamek M, Jablonski M . Alzheimer's mechanisms in ischemic brain degeneration. Anat Rec (Hoboken) 2009; 292: 1863–1881.
Bomboi G, Castello L, Cosentino F, Giubilei F, Orzi F, Volpe M . Alzheimer's disease and endothelial dysfunction. Neurol Sci 2010; 31: 1–8.
Panickar KS, Norenberg MD . Astrocytes in cerebral ischemic injury: morphological and general considerations. Glia 2005; 50: 287–298.
Barreto GE, Gonzalez J, Torres Y, Morales L . Astrocytic-neuronal crosstalk: implications for neuroprotection from brain injury. Neurosci Res 2011; 71: 107–113.
Pekny M, Nilsson M . Astrocyte activation and reactive gliosis. Glia 2005; 50: 427–434.
Ho MC, Lo AC, Kurihara H, Yu AC, Chung SS, Chung SK . Endothelin-1 protects astrocytes from hypoxic/ischemic injury. FASEB J 2001; 15: 618–626.
Yu AC, Wong HK, Yung HW, Lau LT . Ischemia-induced apoptosis in primary cultures of astrocytes. Glia 2001; 35: 121–130.
Pluta R . Astroglial expression of the beta-amyloid in ischemia-reperfusion brain injury. Ann N Y Acad Sci 2002; 977: 102–108.
Li L, Lundkvist A, Andersson D, Wilhelmsson U, Nagai N, Pardo AC et al. Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab 2008; 28: 468–481.
Xu M, Zhang HL . Death and survival of neuronal and astrocytic cells in ischemic brain injury: a role of autophagy. Acta Pharmacol Sin 2011; 32: 1089–1099.
Barres BA . The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 2008; 60: 430–440.
Kaushal V, Schlichter LC . Mechanisms of microglia-mediated neurotoxicity in a new model of the stroke penumbra. J Neurosci 2008; 28: 2221–2230.
Annunziato L, Boscia F, Pignataro G . Ionic transporter activity in astrocytes, microglia, and oligodendrocytes during brain ischemia. J Cereb Blood Flow Metab 2013; 33: 969–982.
Brown GC, Neher JJ . Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol 2010; 41: 242–247.
Schubert P, Ogata T, Marchini C, Ferroni S . Glia-related pathomechanisms in Alzheimer's disease: a therapeutic target? Mech Ageing Dev 2001; 123: 47–57.
Bi X . Alzheimer disease: update on basic mechanisms. J Am Osteopath Assoc 2010; 110: S3–S9.
Thal DR, Schultz C, Dehghani F, Yamaguchi H, Braak H, Braak E . Amyloid beta-protein (Abeta)-containing astrocytes are located preferentially near N-terminal-truncated Abeta deposits in the human entorhinal cortex. Acta Neuropathol 2000; 100: 608–617.
Mukherjee A, Hersh LB . Regulation of amyloid beta-peptide levels by enzymatic degradation. J Alzheimers Dis 2002; 4: 341–348.
Evin G, Sernee MF, Masters CL . Inhibition of gamma-secretase as a therapeutic intervention for Alzheimer's disease: prospects, limitations and strategies. CNS Drugs 2006; 20: 351–372.
Kuo YM, Emmerling MR, Vigo-Pelfrey C, Kasunic TC, Kirpatrick JB, Murdoch GH et al. Water-soluble Abeta (N-40, N-42) oligomers in normal and Alzheimer disease brains. J Biol Chem 1996; 271: 4077–4081.
Roher AE, Chaney MO, Kuo YM, Webster SD, Stine WB, Haverkamp LJ et al. Morphology and toxicity of Abeta-(1-42) dimer derived from neuritic and vascular amyloid deposits of Alzheimer's disease. J Biol Chem 1996; 271: 20631–20635.
Sondag CM, Dhawan G, Combs CK . Beta amyloid oligomers and fibrils stimulate differential activation of primary microglia. J Neuroinflammation 2009; 6: 1.
Dahlgren KN, Manelli AM, Stine WB Jr, Baker LK, Krafft GA, LaDu MJ . Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem 2002; 277: 32046–32053.
Glabe CG, Kayed R . Common structure and toxic function of amyloid oligomers implies a common mechanism of pathogenesis. Neurology 2006; 66: S74–S78.
Itkin A, Dupres V, Dufrêne YF, Bechninger B, Ruysschaert JM, Raussens V . Calcium ions promote formation of amyloid beta-peptide (1-40) oligomers causally implicated in neuronal toxicity of Alzheimer's disease. PLoS One 2011; 6: e18250.
Walsh DM, Tseng BP, Rydel RE, Podlisny MB, Selkoe DJ . The oligomerization of amyloid beta-protein begins intracellularly in cells derived from human brain. Biochemistry 2000; 39: 10831–10839.
Demuro A, Smith M, Parker I . Single-channel Ca(2-) imaging implicates Abeta1-42 amyloid pores in Alzheimer's disease pathology. J Cell Biol 2011; 195: 515–524.
Ferreira IL, Bajouco LM, Mota SI, Auberson YP, Oliveira CR, Rego AC . Amyloid beta peptide 1-42 disturbs intracellular calcium homeostasis through activation of GluN2B-containing N-methyl-d-aspartate receptors in cortical cultures. Cell Calcium 2012; 51: 95–106.
Abramov AY, Canevari L, Duchen MR . Calcium signals induced by amyloid beta peptide and their consequences in neurons and astrocytes in culture. Biochim Biophys Acta 2004; 1742: 81–87.
Bambrick L, Kristian T, Fiskum G . Astrocyte mitochondrial mechanisms of ischemic brain injury and neuroprotection. Neurochem Res 2004; 29: 601–608.
Kokubo H, Kayed R, Glabe CG, Staufenbiel M, Saido TC, Iwata N et al. Amyloid Beta annular protofibrils in cell processes and synapses accumulate with aging and Alzheimer-associated genetic modification. Int J Alzheimers Dis 2009; 2009: 1–7.
Lasagna-Reeves CA, Kayed R . Astrocytes contain amyloid-beta annular protofibrils in Alzheimer's disease brains. FEBS Lett 2011; 585: 3052–3057.
Kayed R, Pensalfini A, Margol L, Sokolov Y, Sarsoza F, Head E et al. Annular protofibrils are a structurally and functionally distinct type of amyloid oligomer. J Biol Chem 2009; 284: 4230–4237.
Volles MJ, Lee SJ, Rochet JC, Shtilerman MD, Ding TT, Kessler JC et al. Vesicle permeabilization by protofibrillar alpha-synuclein: implications for the pathogenesis and treatment of Parkinson's disease. Biochemistry 2001; 40: 7812–7819.
Srinivasan R, Marchant RE, Zagorski MG . ABri peptide associated with familial British dementia forms annular and ring-like protofibrillar structures. Amyloid 2004; 11: 10–13.
Chen M, Zhang S, Liu Q, Liu P, Busuttil K, Wang C et al. An investigation into the formation of annular aggregates of human islet amyloid polypeptide on tantalum oxide surfaces. Chemistry 2012; 18: 2493–2497.
Lasagna-Reeves CA, Glabe CG, Kayed R . Amyloid-beta annular protofibrils evade fibrillar fate in Alzheimer disease brain. J Biol Chem 2011; 286: 22122–22130.
Sojkova J, Zhou Y, An Y, Kraut MA, Ferrucci L, Wong DF et al. Longitudinal patterns of beta-amyloid deposition in nondemented older adults. Arch Neurol 2011; 68: 644–649.
Liu T, Perry G, Chan HW, Verdile G, Martins RN, Smith MA et al. Amyloid-beta-induced toxicity of primary neurons is dependent upon differentiation-associated increases in tau and cyclin-dependent kinase 5 expression. J Neurochem 2004; 88: 554–563.
Ebenezer PJ, Weidner AM, LeVine H 3rd, Markesbery WR, Murphy MP, Zhang L et al. Neuron specific toxicity of oligomeric amyloid-beta: role for JUN-kinase and oxidative stress. J Alzheimers Dis 2010; 22: 839–848.
Lee JT, Xu J, Lee JM, Ku G, Han X, Yang DI et al. Amyloid-beta peptide induces oligodendrocyte death by activating the neutral sphingomyelinase-ceramide pathway. J Cell Biol 2004; 164: 123–131.
Xu J, Chen S, Ahmed SH, Chen H, Ku G, Goldberg MP et al. Amyloid-beta peptides are cytotoxic to oligodendrocytes. J Neurosci 2001; 21: RC118.
Kitazawa M, Yamasaki TR, LaFerla FM . Microglia as a potential bridge between the amyloid beta-peptide and tau. Ann N Y Acad Sci 2004; 1035: 85–103.
Cornejo F, von Bernhardi R . Role of scavenger receptors in glia-mediated neuroinflammatory response associated with Alzheimer's disease. Mediators Inflamm 2013; 2013: 895651.
Nagele RG, D'Andrea MR, Lee H, Venkataraman V, Wang HY . Astrocytes accumulate A beta 42 and give rise to astrocytic amyloid plaques in Alzheimer disease brains. Brain Res 2003; 971: 197–209.
Nielsen HM, Mulder SD, Beliën JA, Musters RJ, Eikelenboom P, Veerhuis R . Astrocytic A beta 1-42 uptake is determined by A beta-aggregation state and the presence of amyloid-associated proteins. Glia 2010; 58: 1235–1246.
Hou L, Liu Y, Wang X, Ma H, He J, Zhang Y et al. The effects of amyloid-beta42 oligomer on the proliferation and activation of astrocytes in vitro. In Vitro Cell Dev Biol Anim 2011; 47: 573–580.
Mulder SD, Veerhuis R, Blankenstein MA, Nielsen HM . The effect of amyloid associated proteins on the expression of genes involved in amyloid-beta clearance by adult human astrocytes. Exp Neurol 2012; 233: 373–379.
Banati RB, Gehrmann J, Wiessner C, Hossmann KA, Kreutzberg GW . Glial expression of the beta-amyloid precursor protein (APP) in global ischemia. J Cereb Blood Flow Metab 1995; 15: 647–654.
Popa-Wagner A, Schroder E, Walker LC, Kessler C . beta-Amyloid precursor protein and ss-amyloid peptide immunoreactivity in the rat brain after middle cerebral artery occlusion: effect of age. Stroke 1998; 29: 2196–2202.
Nihashi T, Inao S, Kajita Y, Kawai T, Sugimoto T, Niwa M et al. Expression and distribution of beta amyloid precursor protein and beta amyloid peptide in reactive astrocytes after transient middle cerebral artery occlusion. Acta Neurochir (Wien) 2001; 143: 287–295.
Linde CI, Baryshnikov SG, Mazzocco-Spezzia A, Golovina VA . Dysregulation of Ca2+ signaling in astrocytes from mice lacking amyloid precursor protein. Am J Physiol Cell Physiol 2011; 300: C1502–C1512.
Hartlage-Rubsamen M, Zeitschel U, Apelt J, Gärtner U, Franke H, Stahl T et al. Astrocytic expression of the Alzheimer's disease beta-secretase (BACE1) is stimulus-dependent. Glia 2003; 41: 169–179.
Zhao J, O'Connor T, Vassar R . The contribution of activated astrocytes to Abeta production: implications for Alzheimer's disease pathogenesis. J Neuroinflammation 2011; 8: 150.
Rossner S, Lange-Dohna C, Zeitschel U, Perez-Polo JR . Alzheimer's disease beta-secretase BACE1 is not a neuron-specific enzyme. J Neurochem 2005; 92: 226–234.
Laws SM, Hone E, Gandy S, Martins RN . Expanding the association between the APOE gene and the risk of Alzheimer's disease: possible roles for APOE promoter polymorphisms and alterations in APOE transcription. J Neurochem 2003; 84: 1215–1236.
Orsitto G, Seripa D, Panza F, Franceschi M, Cascavilla L, Placentino G et al. Apolipoprotein E genotypes in hospitalized elderly patients with vascular dementia. Dement Geriatr Cogn Disord 2007; 23: 327–333.
Qi JP, Wu H, Yang Y, Wang DD, Chen YX, Gu YH et al. Cerebral ischemia and Alzheimer's disease: the expression of amyloid-beta and apolipoprotein E in human hippocampus. J Alzheimers Dis 2007; 12: 335–341.
Yoshimoto S, Ishizaki Y, Mori A, Sasaki T, Takakura K, Murota S . The role of cerebral microvessel endothelium in regulation of cerebral blood flow through production of endothelin-1. J Cardiovasc Pharmacol 1991; 17 ((Suppl 7)): S260–S263.
de Nucci G, Thomas R, D'Orleans-Juste P, Antunes E, Walder C, Warner TD et al. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc Natl Acad Sci USA 1988; 85: 9797–9800.
Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988; 332: 411–415.
Hama H, Kasuya Y, Sakurai T, Yamada G, Suzuki N, Masaki T et al. Role of endothelin-1 in astrocyte responses after acute brain damage. J Neurosci Res 1997; 47: 590–602.
Hasselblatt M, Kamrowski-Kruck H, Jensen N, Schilling L, Kratzin H, Sirén AL et al. ETA and ETB receptor antagonists synergistically increase extracellular endothelin-1 levels in primary rat astrocyte cultures. Brain Res 1998; 785: 253–261.
Cazaubon S, Chaverot N, Romero IA, Girault J-A, Adamson P, Strosberg AD et al. Growth factor activity of endothelin-1 in primary astrocytes mediated by adhesion-dependent and -independent pathways. J Neurosci 1997; 17: 6203–6212.
Gadea A, Schinelli S, Gallo V . Endothelin-1 regulates astrocyte proliferation and reactive gliosis via a JNK/c-Jun signaling pathway. J Neurosci 2008; 28: 2394–2408.
Koyama Y, Baba A, Matsuda T . Endothelins stimulate the expression of neurotrophin-3 in rat brain and rat cultured astrocytes. Neuroscience 2005; 136: 425–433.
Tabernero A, Jimenez C, Velasco A, Giaume C, Medina JM . The enhancement of glucose uptake caused by the collapse of gap junction communication is due to an increase in astrocyte proliferation. J Neurochem 2001; 78: 890–898.
Wang HH, Hsieh HL, Wu CY, Yang CM . Endothelin-1 enhances cell migration via matrix metalloproteinase-9 up-regulation in brain astrocytes. J Neurochem 2010; 113: 1133–1149.
Tykocki NR, Watts SW . The interdependence of endothelin-1 and calcium: a review. Clin Sci (Lond) 2010; 119: 361–372.
Jiang MH, Höög A, Ma KC, Nie XJ, Olsson Y, Zhang WW . Endothelin-1-like immunoreactivity is expressed in human reactive astrocytes. Neuroreport 1993; 4: 935–937.
Lo AC, Chen AY, Hung VK, Yaw LP, Fung MK, Ho MC et al. Endothelin-1 overexpression leads to further water accumulation and brain edema after middle cerebral artery occlusion via aquaporin 4 expression in astrocytic end-feet. J Cereb Blood Flow Metab 2005; 25: 998–1011.
Leung JW, Chung SS, Chung SK . Endothelial endothelin-1 over-expression using receptor tyrosine kinase tie-1 promoter leads to more severe vascular permeability and blood brain barrier breakdown after transient middle cerebral artery occlusion. Brain Res 2009; 1266: 121–129.
Filipovich T, Fleisher-Berkovich S . Regulation of glial inflammatory mediators synthesis: possible role of endothelins. Peptides 2008; 29: 2250–2256.
Luo J, Grammas P . Endothelin-1 is elevated in Alzheimer's disease brain microvessels and is neuroprotective. J Alzheimers Dis 2010; 21: 887–896.
Palmer JC, Baig S, Kehoe PG, Love S . Endothelin-converting enzyme-2 is increased in Alzheimer's disease and up-regulated by Abeta. Am J Pathol 2009; 175: 262–270.
Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med 2003; 9: 907–913.
Kamide T, Kitao Y, Takeishi T, Okada A, Mohri H, Schmidt AM et al. RAGE mediates vascular injury and inflammation after global cerebral ischemia. Neurochem Int 2012; 60: 220–228.
Muhammad S, Barakat W, Stoyanov S, Murikinati S, Yang H, Tracey KJ et al. The HMGB1 receptor RAGE mediates ischemic brain damage. J Neurosci 2008; 28: 12023–12031.
Zhai DX, Kong QF, Xu WS, Bai SS, Peng HS, Zhao K et al. RAGE expression is up-regulated in human cerebral ischemia and pMCAO rats. Neurosci Lett 2008; 445: 117–121.
Taguchi A . Vascular factors in diabetes and Alzheimer's disease. J Alzheimers Dis 2009; 16: 859–864.
Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC et al. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem 1992; 267: 14998–15004.
Han SH, Kim YH, Mook-Jung I . RAGE: the beneficial and deleterious effects by diverse mechanisms of actions. Mol Cells 2011; 31: 91–97.
Xiong F, Leonov S, Howard AC, Xiong S, Zhang B, Mei L et al. Receptor for advanced glycation end products (RAGE) prevents endothelial cell membrane resealing and regulates F-actin remodeling in a beta-catenin-dependent manner. J Biol Chem 2011; 286: 35061–35070.
Takuma K, Fang F, Zhang W, Yan S, Fukuzaki E, Du H et al. RAGE-mediated signaling contributes to intraneuronal transport of amyloid-beta and neuronal dysfunction. Proc Natl Acad Sci U S A 2009; 106: 20021–20026.
Askarova S, Yang X, Sheng W, Sun GY, Lee JC . Role of Abeta-receptor for advanced glycation endproducts interaction in oxidative stress and cytosolic phospholipase A(2) activation in astrocytes and cerebral endothelial cells. Neuroscience 2011; 199: 375–385.
Fang F, Lue LF, Yan S, Xu H, Luddy JS, Chen D et al. RAGE-dependent signaling in microglia contributes to neuroinflammation, Abeta accumulation, and impaired learning/memory in a mouse model of Alzheimer's disease. FASEB J 2010; 24: 1043–1055.
Cho HJ, Son SM, Jin SM, Hong HS, Shin DH, Kim SJ et al. RAGE regulates BACE1 and Abeta generation via NFAT1 activation in Alzheimer's disease animal model. FASEB J 2009; 23: 2639–2649.
Ponath G, Schettler C, Kaestner F, Voigt B, Wentker D, Arolt V et al. Autocrine S100B effects on astrocytes are mediated via RAGE. J Neuroimmunol 2007; 184: 214–222.
Villarreal A, Aviles Reyes RX, Angelo MF, Reines AG, Ramos AJ . S100B alters neuronal survival and dendrite extension via RAGE-mediated NF-kappaB signaling. J Neurochem 2011; 117: 321–332.
Perrone L, Peluso G, Melone MA . RAGE recycles at the plasma membrane in S100B secretory vesicles and promotes Schwann cells morphological changes. J Cell Physiol 2008; 217: 60–71.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Jo, W., Law, A. & Chung, S. The neglected co-star in the dementia drama: the putative roles of astrocytes in the pathogeneses of major neurocognitive disorders. Mol Psychiatry 19, 159–167 (2014). https://doi.org/10.1038/mp.2013.171
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2013.171
Keywords
This article is cited by
-
Cognitive Impairments and blood-brain Barrier Damage in a Mouse Model of Chronic Cerebral Hypoperfusion
Neurochemical Research (2022)
-
Phosphorylation of 5-LOX: The Potential Set-point of Inflammation
Neurochemical Research (2020)
-
Astrocytes are direct cellular targets of lithium treatment: novel roles for lysyl oxidase and peroxisome-proliferator activated receptor-γ as astroglial targets of lithium
Translational Psychiatry (2019)
-
Kainate Receptor Activation Enhances Amyloidogenic Processing of APP in Astrocytes
Molecular Neurobiology (2019)
-
Astrocyte and Alzheimer’s disease
Journal of Neurology (2017)