Abstract
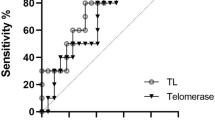
Patients with major depressive disorder (MDD) have an increased onset risk of aging-related somatic diseases such as heart disease, diabetes, obesity and cancer. This suggests mechanisms of accelerated biological aging among the depressed, which can be indicated by a shorter length of telomeres. We examine whether MDD is associated with accelerated biological aging, and whether depression characteristics such as severity, duration, and psychoactive medication do further impact on biological aging. Data are from the Netherlands Study of Depression and Anxiety, including 1095 current MDD patients, 802 remitted MDD patients and 510 control subjects. Telomere length (TL) was assessed as the telomere sequence copy number (T) compared to a single-copy gene copy number (S) using quantitative polymerase chain reaction. This resulted in a T/S ratio and was converted to base pairs (bp). MDD diagnosis and MDD characteristics were determined by self-report questionnaires and structured psychiatric interviews. Compared with control subjects (mean bp=5541), sociodemographic-adjusted TL was shorter among remitted MDD patients (mean bp=5459; P=0.014) and current MDD patients (mean bp=5461; P=0.012). Adjustment for health and lifestyle variables did not reduce the associations. Within the current MDD patients, separate analyses showed that both higher depression severity (P<0.01) and longer symptom duration in the past 4 years (P=0.01) were associated with shorter TL. Our results demonstrate that depressed patients show accelerated cellular aging according to a ‘dose–response’ gradient: those with the most severe and chronic MDD showed the shortest TL. We also confirmed the imprint of past exposure to depression, as those with remitted MDD had shorter TL than controls.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Nicholson A, Kuper H, Hemingway H . Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J 2006; 27: 2763–2774.
Mezuk B, Eaton WW, Albrecht S, Golden SH . Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care 2008; 31: 2383–2390.
Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry 2010; 67: 220–229.
Gao y, Huang C, Zhao K, Ma L, Qiu X, Zhang L et al. Depression as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Int J Geriatr Psychiatry 2012; 28: 441–449.
Chida Y, Hamer M, Wardle J, Steptoe A . Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol 2008; 5: 466–475.
Penninx BW, Guralnik JM, Ferrucci L, Simonsick EM, Deeg DJ, Wallace RB . Depressive symptoms and physical decline in community-dwelling older persons. JAMA 1998; 279: 1720–1726.
Schulz R, Beach SR, Ives DG, Martire LM, Ariyo AA, Kop WJ . Association between depression and mortality in older adults: the Cardiovascular Health Study. Arch Intern Med 2000; 160: 1761–1768.
Cuijpers P, Smit F . Excess mortality in depression: a meta-analysis of community studies. J Affect Disord 2002; 72: 227–236.
Wolkowitz OM, Epel ES, Reus VI, Mellon SH . Depression gets old fast: do stress and depression accelerate cell aging? Depress Anxiety 2010; 27: 327–338.
Wolkowitz OM, Reus VI, Mellon SH . Of sound mind and body: depression, disease, and accelerated aging. Dialogues Clin Neurosci 2011; 13: 25–39.
Blackburn EH . Switching and signaling at the telomere. Cell 2001; 106: 661–673.
Epel ES . Psychological and metabolic stress: a recipe for accelerated cellular aging? Hormones (Athens) 2009; 8: 7–22.
Olovnikov AM . Telomeres, telomerase, and aging: origin of the theory. Exp Gerontol 1996; 31: 443–448.
Blackburn EH, Greider CW, Szostak JW . Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med 2006; 12: 1133–1138.
Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP et al. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol 2007; 165: 14–21.
Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF et al. Obesity, cigarette smoking, and telomere length in women. Lancet 2005; 366: 662–664.
Sampson MJ, Winterbone MS, Hughes JC, Dozio N, Hughes DA . Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care 2006; 29: 283–289.
Willeit P, Willeit J, Mayr A, Weger S, Oberhollenzer F, Brandstätter A et al. Telomere length and risk of incident cancer and cancer mortality. JAMA 2010; 304: 69–75.
Martin-Ruiz C, Dickinson HO, Keys B, Rowan E, Kenny RA, von Zglinicki T . Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann Neurol 2006; 60: 174–180.
Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA . Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 2003; 361: 393–395.
Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH et al. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry 2006; 60: 432–435.
Lung FW, Chen NC, Shu BC . Genetic pathway of major depressive disorder in shortening telomeric length. Psychiatr Genet 2007; 17: 195–199.
Hartmann N, Boehner M, Groenen F, Kalb R . Telomere length of patients with major depression is shortened but independent from therapy and severity of the disease. Depress Anxiety 2010; 27: 1111–1116.
Hoen PW, de Jonge P, Na BY, Farzaneh-Far R, Epel E, Lin J et al. Depression and leukocyte telomere length in patients with coronary heart disease: data from the Heart and Soul Study. Psychosom Med 2011; 73: 541–547.
Wolkowitz OM, Mellon SH, Epel ES, Lin J, Dhabhar FS, Su Y et al. Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress—preliminary findings. PLoS One 2011; 6: e17837.
Wikgren M, Maripuu M, Karlsson T, Nordfjäll K, Bergdahl J, Hultdin J et al. Short telomeres in depression and the general population are associated with a hypocortisolemic state. Biol Psychiatry 2012; 71: 294–300.
Penninx BWJH, Beekman ATF, Smit JH, Zitman FG, Nolen WA, Spinhoven P et al. The Netherlands Study of Depression and Anxiety (NESDA): rationale, objectives and methods. Int J Methods Psychiatr Res 2008; 17: 121–140.
Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH . The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med 1996; 26: 477–486.
Cawthon RM . Telomere measurement by quantitative PCR. Nucleic Acids Res 2002; 30: e47.
Aviv A, Hunt SC, Lin J, Cao X, Kimura M, Blackburn E . Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res 2011; 39: e134.
Lin J, Epel E, Cheon J, Kroenke C, Sinclair E, Bigos M et al. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J Immunol Methods 2010; 352: 71–80.
Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003; 54: 573–583.
Lyketsos CG, Nestadt G, Cwi J, Heithoff K . The life-chart interview: a standardized method to describe the course of psychopathology. Int J Meth Psychiatr Res 1994; 4: 143–155.
de Graaf R, Bijl RV, Smit F, Vollebergh WAM, Spijker J . Risk factors for 12-month comorbidity of mood, anxiety, and substance use disorders: findings from the Netherlands Mental Health Survey and Incidence Study. Am J Psychiatry 2002; 159: 620–629.
Hovens JG, Wiersma JE, Giltay EJ, van Oppen P, Spinhoven P, Penninx BW et al. Childhood life events and childhood trauma in adult patients with depressive, anxiety and comorbid disorders vs. controls. Acta Psychiatr Scand 2010; 122: 66–74.
Wiersma JE, Hovens JG, van OP, Giltay EJ, van Schaik DJ, Beekman AT et al. The importance of childhood trauma and childhood life events for chronicity of depression in adults. J Clin Psychiatry 2009; 70: 983–989.
Spinhoven P, Elzinga BM, Hovens JG, Roelofs K, Zitman FG, van Oppen P et al. The specificity of childhood adversities and negative life events across the life span to anxiety and depressive disorders. J Affect Disord 2010; 126: 103–112.
World Health Organization Collaboration Centre for Drug Statistics Methodology. Anatomical Therapeutic Chemical (ATC) Classification System. World Health Organization Collaboration Centre for Drug Statistics Methodology: Oslo, Norway, 2007.
Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003; 35: 1381–1395.
Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor-Locke C et al. 2011 Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc 2011; 43: 1575–1581.
Howren MB, Lamkin DM, Suls J . Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 2009; 71: 171–186.
Molendijk M, Bus B, Oude Voshaar R, Spinhoven P, Penninx BW, Elzinga B . Serum levels of brain-derived neurotrophic factor in major depressive disorder: state—trait issues, clinical features, and pharmacological treatment. Mol Psychiatry 2010; 16: 1088–1095.
Vreeburg SA, Hoogendijk WJ, van PJ, Derijk RH, Verhagen JC, van Dyck R et al. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry 2009; 66: 617–626.
Price LH, Kao HT, Burgers DE, Carpenter LL, Tyrka AR . Telomeres and early-life stress: an overview. Biol Psychiatry 2013; 73: 15–23.
Penninx BW, Beekman AT, Bandinelli S, Corsi AM, Bremmer M, Hoogendijk WJ et al. Late-life depressive symptoms are associated with both hyperactivity and hypoactivity of the hypothalamo-pituitary-adrenal axis. Am J Geriatr Psychiatry 2007; 15: 522–529.
Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK et al. A meta-analysis of cytokines in major depression. Biol Psychiatry 2010; 67: 446–457.
Vogelzangs N, Duivis HE, Beekman ATF, Kluft C, Neuteboom J, Hoogendijk W et al. Association of depressive disorders, depression characteristics and antidepressant medication with inflammation. Transl Psychiatry 2012; 2: e79.
Rottenberg J . Cardiac vagal control in depression: a critical analysis. Biol Psychol 2007; 74: 200–211.
Licht CM, de Geus EJ, Zitman FG, Hoogendijk WJ, van DR, Penninx BW . Association between major depressive disorder and heart rate variability in the Netherlands Study of Depression and Anxiety (NESDA). Arch Gen Psychiatry 2008; 65: 1358–1367.
Michel TM, Pulschen D, Thome J . The role of oxidative stress in depressive disorders. Curr Pharm Des 2012; 18: 5890–5899.
Choi J, Fauce SR, Effros RB . Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav Immun 2008; 22: 600–605.
von Zglinicki T . Oxidative stress shortens telomeres. Trends Biochem Sci 2002; 27: 339–344.
Damjanovic AK, Yang Y, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B et al. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer's disease patients. J Immunol 2007; 179: 4249–4254.
Aviv A, Chen W, Gardner JP, Kimura M, Brimacombe M, Cao X et al. Leukocyte telomere dynamics: longitudinal findings among young adults in the Bogalusa Heart Study. Am J Epidemiol 2009; 169: 323–329.
Nordfjall K, Svenson U, Norrback KF, Adolfsson R, Lenner P, Roos G . The individual blood cell telomere attrition rate is telomere length dependent. PLoS Genet 2009; 5: e1000375.
Shalev I, Moffitt T, Sugden K, Williams B, Houts RM, Danese A et al. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Mol Psychiatry 2012; 18: 576–581.
Teyssier JR, Ragot S, Donzel A, Chauvet-Gelinier JC . [Telomeres in the brain cortex of depressive patients]. Encephale 2010; 36: 491–494.
Zhang D, Cheng L, Craig DW, Redman M, Liu C . Cerebellar telomere length and psychiatric disorders. Behav Genet 2010; 40: 250–254.
Zhou QG, Hu Y, Wu DL, Zhu LJ, Chen C, Jin X et al. Hippocampal telomerase is involved in the modulation of depressive behaviors. J Neurosci 2011; 31: 12258–12269.
Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA 2004; 101: 17312–17315.
Wolkowitz OM, Mellon SH, Epel ES, Lin J, Reus VI, Rosser R et al. Resting leukocyte telomerase activity is elevated in major depression and predicts treatment response. Mol Psychiatry 2012; 17: 164–172.
Puterman E, Lin J, Blackburn E, O'Donovan A, Adler N, Epel E . The power of exercise: buffering the effect of chronic stress on telomere length. PLoS One 2010; 5: e10837.
Du M, Prescott J, Kraft P, Han J, Giovannucci E, Hankinson SE et al. Physical activity, sedentary behavior, and leukocyte telomere length in women. Am J Epidemiol 2012; 175: 414–422.
Lin J, Epel E, Blackburn E . Telomeres and lifestyle factors: roles in cellular aging. Mutat Res 2012; 730: 85–89.
Osthus IB, Sgura A, Berardinelli F, Alsnes IV, Brønstad E, Rehn T et al. Telomere length and long-term endurance exercise: does exercise training affect biological age? A pilot study. PLoS One 2012; 7: e52769.
Acknowledgements
The infrastructure for the NESDA study (www.nesda.nl) is funded through the Geestkracht program of the Netherlands Organization for Health Research and Development (Zon-Mw, grant number 10-000-1002) and is supported by participating universities and mental health-care organizations (VU University Medical Center, GGZ inGeest, Arkin, Leiden University Medical Center, GGZ Rivierduinen, University Medical Center Groningen, Lentis, GGZ Friesland, GGZ Drenthe, Institute for Quality of Health Care (IQ Healthcare), Netherlands Institute for Health Services Research (NIVEL) and Netherlands Institute of Mental Health and Addiction (Trimbos)). BP, JV, DR and telomere length assaying were supported through a NWO-VICI grant (number 91811602).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
EE is a co-founder of Telome Health, Inc, a telomere measurement company. JL is an Associate Research Biochemist in the Department of Biochemistry and Biophysics at UCSF. The other authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
PowerPoint slides
Rights and permissions
About this article
Cite this article
Verhoeven, J., Révész, D., Epel, E. et al. Major depressive disorder and accelerated cellular aging: results from a large psychiatric cohort study. Mol Psychiatry 19, 895–901 (2014). https://doi.org/10.1038/mp.2013.151
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2013.151
Keywords
This article is cited by
-
Clinical correlates and thyroid hormones of metabolic syndrome in first-episode and drug-naïve major depressive disorder outpatients with and without hyperglycemia: a comprehensive cross-sectional study
BMC Psychiatry (2023)
-
Strong associations of telomere length and mitochondrial copy number with suicidality and abuse history in adolescent depressed individuals
Molecular Psychiatry (2023)
-
Shorter telomere length predicts poor antidepressant response and poorer cardiometabolic indices in major depression
Scientific Reports (2023)
-
Associations Between Children’s Telomere Length, Parental Intrusiveness, and the Development of Early Externalizing Behaviors
Child Psychiatry & Human Development (2023)
-
A healthy lifestyle is positively associated with mental health and well-being and core markers in ageing
BMC Medicine (2022)