Abstract
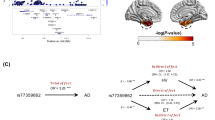
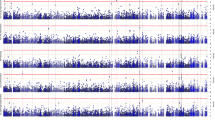
Prior to intervention trials in individuals genetically at-risk for late-onset Alzheimer’s disease, critical first steps are identifying where (neuroanatomic effects), when (timepoint in the lifespan) and how (gene expression and neuropathology) Alzheimer’s risk genes impact the brain. We hypothesized that variants in the sortilin-like receptor (SORL1) gene would affect multiple Alzheimer’s phenotypes before the clinical onset of symptoms. Four independent samples were analyzed to determine effects of SORL1 genetic risk variants across the lifespan at multiple phenotypic levels: (1) microstructural integrity of white matter using diffusion tensor imaging in two healthy control samples (n=118, age 18–86; n=68, age 8–40); (2) gene expression using the Braincloud postmortem healthy control sample (n=269, age 0–92) and (3) Alzheimer’s neuropathology (amyloid plaques and tau tangles) using a postmortem sample of healthy, mild cognitive impairment (MCI) and Alzheimer’s individuals (n=710, age 66–108). SORL1 risk variants predicted lower white matter fractional anisotropy in an age-independent manner in fronto-temporal white matter tracts in both samples at 5% family-wise error-corrected thresholds. SORL1 risk variants also predicted decreased SORL1 mRNA expression, most prominently during childhood and adolescence, and significantly predicted increases in amyloid pathology in postmortem brain. Importantly, the effects of SORL1 variation on both white matter microstructure and gene expression were observed during neurodevelopmental phases of the human lifespan. Further, the neuropathological mechanism of risk appears to primarily involve amyloidogenic pathways. Interventions targeted toward the SORL1 amyloid risk pathway may be of greatest value during early phases of the lifespan.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Prince M, Jackson J Alzheimer’s Disease International World Alzheimer Report 2009. Alzheimer’s Disease International, 2009 (cited 2012). Available fromhttp://www.alz.co.uk/research/files/WorldAlzheimerReport.pdf.
Fox N . When, where, and how does Alzheimer’s disease start? Lancet Neurol 2012; 11: 1017–1018.
Reiman EM, Quiroz YT, Fleisher AS, Chen K, Velez-Pardo C, Jimenez-Del-Rio M et al. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer’s disease in the presenilin 1 E280A kindred: a case-control study. Lancet Neurol 2012; 11: 1048–1056.
Reiman EM, Langbaum JBS, Tariot PN . Alzheimer’s prevention initiative: a proposal to evaluate presymptomatic treatments as quickly as possible. Biomark Med 2010; 4: 3–14.
Felsky D, Voineskos AN . APoe ε 4, aging, and effects on white matter across the adult life span. JAMA Psychiatry 2013; 70: 646–647.
Trachtenberg AJ, Filippini N, Mackay CE . The effects of APOE-ɛ4 on the BOLD response. Neurobiol Aging 2012; 33: 323–334.
Bertram L, Tanzi RE . The genetics of Alzheimer’s disease. Prog Mol Biol Transl Sci 2012; 107: 79–100.
Hoe H-S, Rebeck GW . Functional interactions of APP with the apoE receptor family. J Neurochem 2008; 106: 2263–2271.
Andersen OM, Reiche J, Schmidt V, Gotthardt M, Spoelgen R, Behlke J et al. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc Natl Acad Sci USA 2005; 102: 13461–13466.
Offe K, Dodson SE, Shoemaker JT, Fritz JJ, Gearing M, Levey AI et al. The lipoprotein receptor LR11 regulates amyloid beta production and amyloid precursor protein traffic in endosomal compartments. J Neurosci 2006; 26: 1596–1603.
Capsoni S, Carlo A-S, Vignone D, Amato G, Criscuolo C, Willnow TE et al. SorLA deficiency dissects amyloid pathology from tau and cholinergic neurodegeneration in a mouse model of Alzheimer’s disease. J Alzheimers Dis 2012; 33: 357–371.
Klinger SC, Glerup S, Raarup MK, Mari MC, Nyegaard M, Koster G et al. SorLA regulates the activity of lipoprotein lipase by intracellular trafficking. J Cell Sci 2011; 124: 1095–1105.
Lee JH, Barral S, Reitz C . The neuronal sortilin-related receptor gene SORL1 and late-onset Alzheimer’s disease. Curr Neurol Neurosci Rep 2008; 8: 384–391.
Reitz C, Cheng R, Rogaeva E, Lee JH, Tokuhiro S, Zou F et al. Meta-analysis of the association between variants in SORL1 and Alzheimer disease. Arch Neurol 2011; 68: 99–106.
Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, Zou F et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet 2007; 39: 168–177.
Pottier C, Hannequin D, Coutant S, Rovelet-Lecrux A, Wallon D, Rousseau S et al. High frequency of potentially pathogenic SORL1 mutations in autosomal dominant early-onset Alzheimer disease. Mol Psychiatr 2012; 17: 875–879.
Caglayan S, Bauerfeind A, Schmidt V, Carlo A-S, Prabakaran T, Hübner N et al. Identification of Alzheimer disease risk genotype that predicts efficiency of SORL1 expression in the brain. Arch Neurol 2012; 69: 373–379.
Grear KE, Ling I-F, Simpson JF, Furman JL, Simmons CR, Peterson SL et al. Expression of SORL1 and a novel SORL1 splice variant in normal and Alzheimers disease brain. Mol Neurodegener 2009; 4: 46.
Sager KL, Wuu J, Leurgans SE, Rees HD, Gearing M, Mufson EJ et al. Neuronal LR11/sorLA expression is reduced in mild cognitive impairment. Ann Neurol 2007; 62: 640–647.
Scherzer CR, Offe K, Gearing M, Rees HD, Fang G, Heilman CJ et al. Loss of apolipoprotein E receptor LR11 in Alzheimer disease. Arch Neurol 2004; 61: 1200–1205.
Cuenco KT, Lunetta KL, Baldwin CT, McKee AC, Guo J, Cupples LA et al. Association of distinct variants in SORL1 with cerebrovascular and neurodegenerative changes related to Alzheimer disease. Arch Neurol 2008; 65: 1640–1648.
Bralten J, Arias-Vásquez A, Makkinje R, Veltman JA, Brunner HG, Fernández G et al. Association of the Alzheimer’s gene SORL1 with hippocampal volume in young, healthy adults. Am J Psychiatry 2011; 168: 1083–1089.
Zhuang L, Sachdev PS, Trollor JN, Kochan NA, Reppermund S, Brodaty H et al. Microstructural white matter changes in cognitively normal individuals at risk of amnestic MCI. Neurology 2012; 79: 748–754.
Folstein MF, Folstein SE, McHugh PR . ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–198.
Voineskos AN, O’Donnell LJ, Lobaugh NJ, Markant D, Ameis SH, Niethammer M et al. Quantitative examination of a novel clustering method using magnetic resonance diffusion tensor tractography. Neuroimage 2009; 45: 370–376.
Felsky D, Voineskos AN . APOE ɛ4, aging, and effects on white matter across the adult lifespan. Arch Gen Psychiatry 2013; 70: 646–647.
Felsky D, Voineskos AN, Lerch JP, Nazeri A, Shaikh SA, Rajji TK et al. Myelin-associated glycoprotein gene and brain morphometry in schizophrenia. Front Psychiatry 2012; 3: 40.
Westlye LT, Walhovd KB, Dale AM, Bjørnerud A, Due-Tønnessen P, Engvig A et al. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb Cortex 2010; 20: 2055–2068.
Peters BD, Szeszko PR, Radua J, Ikuta T, Gruner P, DeRosse P et al. White matter development in adolescence: diffusion tensor imaging and meta-analytic results. Schizophr Bull 2012; 38: 1308–1317.
Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36: 980–988.
First MB, Spitzer RL, Gibbon M, Williams JBW Structured clinical interview for DSM-IV-TR Axis I disorders, research version, non-patient edition. (SCID-I/NP) 2002.
Lipska BK, Deep-Soboslay A, Weickert CS, Hyde TM, Martin CE, Herman MM et al. Critical factors in gene expression in postmortem human brain: focus on studies in schizophrenia. Biological Psychiatry 2006; 60: 650–658.
Colantuoni C, Lipska BK, Ye T, Hyde TM, Tao R, Leek JT et al. Temporal dynamics and genetic control of transcription in the human prefrontal cortex. Nature 2011; 478: 519–523.
Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS . Overview and findings from the religious orders study. Curr Alzheimer Res 2012; 9: 628–645.
Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS . Overview and findings from the rush Memory and Aging Project. Curr Alzheimer Res 2012; 9: 646–663.
Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah RC, Kelly JF et al. Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology 2006; 27: 169–176.
Bennett DA, Wilson RS, Boyle PA, Buchman AS, Schneider JA . Relation of neuropathology to cognition in persons without cognitive impairment. Ann Neurol 2012; 72: 599–609.
Chibnik LB, Shulman JM, Leurgans SE, Schneider JA, Wilson RS, Tran D et al. CR1 is associated with amyloid plaque burden and age-related cognitive decline. Ann Neurol 2011; 69: 560–569.
Schroeder A, Mueller O, Stocker S, Salowsky R, Leiber M, Gassmann M et al. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol 2006; 7: 3.
Kimura R, Yamamoto M, Morihara T, Akatsu H, Kudo T, Kamino K et al. SORL1 is genetically associated with Alzheimer disease in a Japanese population. Neurosci Lett 2009; 461: 177–180.
Tan EK, Lee J, Chen CP, Teo YY, Zhao Y, Lee WL . SORL1 haplotypes modulate risk of Alzheimer’s disease in Chinese. Neurobiol Aging 2009; 30: 1048–1051.
Feulner TM, Laws SM, Friedrich P, Wagenpfeil S, Wurst SHR, Riehle C et al. Examination of the current top candidate genes for AD in a genome-wide association study. Mol Psychiatry 2010; 15: 756–766.
Meng Y, Lee JH, Cheng R St, George-Hyslop P, Mayeux R, Farrer LA . Association between SORL1 and Alzheimer’s disease in a genome-wide study. Neuroreport 2007; 18: 1761–1764.
De Leon MJ, DeSanti S, Zinkowski R, Mehta PD, Pratico D, Segal S et al. MRI and CSF studies in the early diagnosis of Alzheimer’s disease. J Intern Med 2004; 256: 205–223.
Jack CR Jr, Shiung MM, Gunter JL, O’Brien PC, Weigand SD, Knopman DS et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology 2004; 62: 591–600.
Karas GB, Scheltens P, Rombouts SARB, Visser PJ, van Schijndel RA, Fox NC et al. Global and local gray matter loss in mild cognitive impairment and Alzheimer’s disease. Neuroimage 2004; 23: 708–716.
Huang H, Fan X, Weiner M, Martin-Cook K, Xiao G, Davis J et al. Distinctive disruption patterns of white matter tracts in Alzheimer’s disease with full diffusion tensor characterization. Neurobiology Aging 2012; 33: 2029–2045.
Pievani M, Agosta F, Pagani E, Canu E, Sala S, Absinta M et al. Assessment of white matter tract damage in mild cognitive impairment and Alzheimer’s disease. Hum Brain Map 2010; 31: 1862–1875.
Zhang Y, Schuff N, Du A-T, Rosen HJ, Kramer JH, Gorno-Tempini ML et al. White matter damage in frontotemporal dementia and Alzheimer’s disease measured by diffusion MRI. Brain 2009; 132: 2579–2592.
Carmichael OT, Salloway S . Imaging markers of incipient dementia The white matter matters. Neurology 2012; 79: 726–727.
Hopkins RO, Beck CJ, Burnett DL, Weaver LK, Victoroff J, Bigler ED . Prevalence of white matter hyperintensities in a young healthy population. J Neuroimag 2006; 16: 243–251.
McCarthy JJ, Saith S, Linnertz C, Burke JR, Hulette CM, Welsh-Bohmer KA et al. The Alzheimer’s associated 5’ region of the SORL1 gene cis regulates SORL1 transcripts expression. Neurobiol Aging 2012; 33: 1485, e1–8.
Schmidt V, Baum K, Lao A, Rateitschak K, Schmitz Y, Teichmann A et al. Quantitative modelling of amyloidogenic processing and its influence by SORLA in Alzheimer’s disease. EMBO J 2012; 31: 187–200.
Ma Q-L, Galasko DR, Ringman JM, Vinters HV, Edland SD, Pomakian J et al. Reduction of SorLA/LR11, a sorting protein limiting beta-amyloid production, in Alzheimer disease cerebrospinal fluid. Arch Neurol 2009; 66: 448–457.
Dodson SE, Gearing M, Lippa CF, Montine TJ, Levey AI, Lah JJ . LR11/SorLA expression is reduced in sporadic Alzheimer disease but not in familial Alzheimer disease. J Neuropathol Exp Neurol 2006; 65: 866–872.
Cruchaga C, Kauwe JSK, Mayo K, Spiegel N, Bertelsen S, Nowotny P et al. SNPs associated with cerebrospinal fluid phospho-tau levels influence rate of decline in Alzheimer’s disease. PLoS Genet 2010; 6: e1001101.
Alexopoulos P, Guo L-H, Tsolakidou A, Kratzer M, Grimmer T, Westerteicher C et al. Interrelations between CSF soluble AβPPβ, amyloid-β 1-42, SORL1, and tau levels in Alzheimer’s disease. J Alzheimers Dis 2012; 28: 543–552.
Bendlin BB, Carlsson CM, Johnson SC, Zetterberg H, Blennow K, Willette AA et al. CSF T-Tau/Aβ42 predicts white matter microstructure in healthy adults at risk for Alzheimer’s disease. PLoS One 2012; 7: e37720.
White T, Nelson M, Lim KO . Diffusion tensor imaging in psychiatric disorders. Top Magn Reson Imaging 2008; 19: 97–109.
Zhang Y, Schuff N, Jahng G-H, Bayne W, Mori S, Schad L et al. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology 2007; 68: 13–19.
Larroza A, Moratal D, D’ocón Alcañiz V, Arana E . por la Alzheimer’s Disease Neuroimaging Initiative. Tractography of the uncinate fasciculus and the posterior cingulate fasciculus in patients with mild cognitive impairment and Alzheimer disease. Neurologia, advance online publication, 10 April 2013; pii: S0213-4853(13)00022-4; doi:10.1016/j.nrl.2013.02.002 (e-pub ahead of print).
Morikawa M, Kiuchi K, Taoka T, Nagauchi K, Kichikawa K, Kishimoto T . Uncinate fasciculus-correlated cognition in Alzheimer’s disease: a diffusion tensor imaging study by tractography. Psychogeriatrics 2010; 10: 15–20.
Wang J-H, Lv P-Y, Wang H-B, Li Z-L, Li N, Sun Z-Y et al. Diffusion tensor imaging measures of normal appearing white matter in patients who are aging, or have amnestic mild cognitive impairment, or Alzheimer’s disease. J Clin Neurosci 2013; 20: 1089–1094.
Acknowledgements
The BrainCloud postmortem data used for the analysis described in this manuscript were obtained from dbGaP at http://www.ncbi.nlm.nih.gov/gap through dbGaP accession number phs000417.v1.p1. Submission of the data, phs000417.v1.p1 to dbGaP was provided by Drs Barbara Lipska and Joel Kleinman. Collection of the data was through a collaborative study sponsored by the NIMH Intramural Research Program. Initial report on this data set is from Colantuoni et al.33 We would also like to thank all of the study participants and acknowledge the essential contributions of Chaya Gopin and Kimberly Cameron to the recruitment and clinical assessments of those participants. We are indebted to the participants in the Religious Orders Study and the Rush Memory and Aging Project. We thank the staff of the Rush Alzheimer’s Disease Center. Work from Rush was supported in part by grants P30AG10161, R01AG15819, R01AG17917, R01AG30146, the Illinois Department of Public Health and the Translational Genomics Research Institute. Work from Hillside was supported by NIMH grant P50MH080173. Work from CAMH was supported in part by the CAMH Foundation thanks to the Kimel Family, Koerner New Scientist Award, and Paul E Garfinkel New Investigator Catalyst Award, as well as the Canadian Institutes of Health Research, Ontario Mental Health Foundation, the Alzheimer’s Society of Canada, and NIMH grant R01MH099167.
Disclaimer
No sponsor or funder had any role in the design and conduct of the study, collection, management, analysis and interpretation of the data, and preparation, review or approval of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Within the past 5 years, BGP has been a member of the advisory board of Lundbeck Canada (final meeting was May 2009) and Forest Laboratories (final meeting was March 2008). He has also served one time as a consultant for Wyeth (October 2008) and Takeda (July 2007) and was a faculty member of the Lundbeck International Neuroscience Foundation (LINF) (final meeting was April 2010). JLK has been a consultant to GlaxoSmithKline, Sanofi-Aventis and Dianippon-Sumitomo. BHM has received travel support from Roche. AKM has served as a consultant for Genomind Inc. All other authors declare no conflict of interest. ANV had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
PowerPoint slides
Rights and permissions
About this article
Cite this article
Felsky, D., Szeszko, P., Yu, L. et al. The SORL1 gene and convergent neural risk for Alzheimer’s disease across the human lifespan. Mol Psychiatry 19, 1125–1132 (2014). https://doi.org/10.1038/mp.2013.142
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2013.142
Keywords
This article is cited by
-
Lack of human-like extracellular sortilin neuropathology in transgenic Alzheimer’s disease model mice and macaques
Alzheimer's Research & Therapy (2018)
-
Effect of Alzheimer's Disease Risk Variant rs3824968 at SORL1 on Regional Gray Matter Volume and Age-Related Interaction in Adult Lifespan
Scientific Reports (2016)
-
Modulation effect of the SORL1 gene on functional connectivity density in healthy young adults
Brain Structure and Function (2016)
-
Risk factor SORL1: from genetic association to functional validation in Alzheimer’s disease
Acta Neuropathologica (2016)
-
Sex Moderates the Effects of the Sorl1 Gene rs2070045 Polymorphism on Cognitive Impairment and Disruption of the Cingulum Integrity in Healthy Elderly
Neuropsychopharmacology (2015)