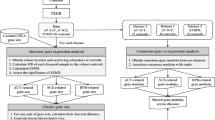
Abstract
Schizophrenia (SCZ) and bipolar disorder (BD) are highly heritable psychiatric disorders. Associated genetic and gene expression changes have been identified, but many have not been replicated and have unknown functions. We identified groups of genes whose expressions varied together, that is co-expression modules, then tested them for association with SCZ. Using weighted gene co-expression network analysis, we show that two modules were differentially expressed in patients versus controls. One, upregulated in cerebral cortex, was enriched with neuron differentiation and neuron development genes, as well as disease genome-wide association study genetic signals; the second, altered in cerebral cortex and cerebellum, was enriched with genes involved in neuron protection functions. The findings were preserved in five expression data sets, including sets from three brain regions, from a different microarray platform, and from BD patients. From those observations, we propose neuron differentiation and development pathways may be involved in etiologies of both SCZ and BD, and neuron protection function participates in pathological process of the diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jablensky A . The 100-year epidemiology of schizophrenia. Schizophr Res 1997; 28: 111–125.
McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A . The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry 2003; 60: 497–502.
Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RMA, Petukhova M et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the national comorbidity survey replication. Arch Gen Psychiatry 2007; 64: 543–552.
McGrath J, Saha S, Chant D, Welham J . Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev 2008; 30: 67–76.
Burmeister M, McInnis MG, Zollner S . Psychiatric genetics: progress amid controversy. Nat Rev Genet 2008; 9: 527–540.
Sanders J, Gill M . Unravelling the genome: a review of molecular genetic research in schizophrenia. Ir J Med Sci 2007; 176: 5–9.
Hattori E, Liu CY, Badner JA, Bonner TI, Christian SL, Maheshwari M et al. Polymorphisms at the G72/G30 gene locus, on 13q33, are associated with bipolar disorder in two independent pedigree series. Am J Hum Genet 2003; 72: 1131–1140.
O'Donovan MC, Williams HJ, Owen MJ . New findings from genetic association studies of schizophrenia. J Hum Genet 2009; 54: 9–14.
Shi LM, Campbell G, Jones WD, Campagne F, Wen ZN, Walker SJ et al. The MicroArray Quality Control (MAQC)-II study of common practices for the development and validation of microarray-based predictive models. Nat Biotechnol 2010; 28: 827–838.
Shi LM, Reid LH, Jones WD, Shippy R, Warrington JA, Baker SC et al. The MicroArray Quality Control (MAQC) project shows inter- and intraplatform reproducibility of gene expression measurements. Nat Biotechnol 2006; 24: 1151–1161.
Sequeira PA, Martin MV, Vawter MP . The first decade and beyond of transcriptional profiling in schizophrenia. Neurobiol Dis 2011; 45: 23–36.
Horvath S, Langfelder P . WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 2008; 9: 559.
Zhang B, Horvath S . A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol 2005; 4, Article17.
Cai CC, Langfelder P, Fuller TF, Oldham MC, Luo R, van den Berg LH et al. Is human blood a good surrogate for brain tissue in transcriptional studies? BMC Genomics 2010; 11: 589.
Fuller TF, Ghazalpour A, Aten JE, Drake TA, Lusis AJ, Horvath S . Weighted gene coexpression network analysis strategies applied to mouse weight. Mamm Genome 2007; 18: 463–472.
Kang HJ, Kawasawa YI, Cheng F, Zhu Y, Xu XM, Li MF et al. Spatio-temporal transcriptome of the human brain. Nature 2011; 478: 483–489.
Oldham MC, Horvath S, Geschwind DH . Conservation and evolution of gene co-expression networks in human and chimpanzee brains. Proc Natl Acad Sci USA 2006; 103: 17973–17978.
Oldham MC, Konopka G, Iwamoto K, Langfelder P, Kato T, Horvath S et al. Functional organization of the transcriptome in human brain. Nat Neurosci 2008; 11: 1271–1282.
Torkamani A, Dean B, Schork NJ, Thomas EA . Coexpression network analysis of neural tissue reveals perturbations in developmental processes in schizophrenia. Genome Res 2010; 20: 403–412.
Geschwind DH, Voineagu I, Wang XC, Johnston P, Lowe JK, Tian Y et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 2011; 474: 380–384.
Ripke S, Sanders AR, Kendler KS, Levinson DF, Sklar P, Holmans PA et al. Genome-wide association study identifies five new schizophrenia loci. Nat Genet 2011; 43: 969–976.
Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, Sullivan PF et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 2009; 460: 748–752.
Shi JX, Levinson DF, Duan JB, Sanders AR, Zheng YL, Pe'er I et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature 2009; 460: 753–757.
Goes FS, Zandi PP, Miao K, McMahon FJ, Steele J, Willour VL et al. Mood-incongruent psychotic features in bipolar disorder: familial aggregation and suggestive linkage to 2p11-q14 and 13q21-33. Am J Psychiatry 2007; 164: 236–247.
Torrey EF, Webster M, Knable M, Johnston N, Yolken RH . The Stanley Foundation brain collection and Neuropathology Consortium. Schizophr Res 2000; 44: 151–155.
Narayan S, Tang B, Head SR, Gilmartin TJ, Sutcliffe JG, Dean B et al. Molecular profiles of schizophrenia in the CNS at different stages of illness. Brain Res 2008; 1239: 235–248.
Chen C, Grennan K, Badner J, Zhang DD, Gershon E, Jin L et al. Removing batch effects in analysis of expression microarray data: an evaluation of six batch adjustment methods. PLoS One 2011; 6: e17238.
Johnson WE, Li C, Rabinovic A . Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2007; 8: 118–127.
Langfelder P, Zhang B, Horvath S . Defining clusters from a hierarchical cluster tree: the Dynamic Tree Cut package for R. Bioinformatics 2008; 24: 719–720.
Langfelder P, Horvath S . Eigengene networks for studying the relationships between co-expression modules. BMC Syst Biol 2007; 1: 54.
Langfelder P, Luo R, Oldham MC, Horvath S . Is my network module preserved and reproducible? Plos Comput Biol 2011; 7: e1001057.
Storey JD, Tibshirani R . Statistical significance for genome-wide studies. Proc Natl Acad Sci USA 2003; 100: 3889–3894.
Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR . MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol 2010; 34: 816–834.
Wang J, Zhang KL, Cui SJ, Chang SH, Zhang LY . i-GSEA4GWAS: a web server for identification of pathways/gene sets associated with traits by applying an improved gene set enrichment analysis to genome-wide association study. Nucleic Acids Res 2010; 38: W90–W95.
Peilin J, Tian J, Zhao Z . Assessing gene length biases in gene set analysis of Genome-Wide Association Studies. Int J Comput Biol Drug Design 2010; 3: 297–310.
Jia P, Wang L, Fanous AH, Chen X, Kendler KS et alInternational Schizophrenia Consortium. A bias-reducing pathway enrichment analysis of genome-wide association data confirmed association of the MHC region with schizophrenia. J Med Genet 2012; 49: 96–10.
Thomas EA, Torkamani A, Dean B, Schork NJ . Coexpression network analysis of neural tissue reveals perturbations in developmental processes in schizophrenia. Genome Res 2010; 20: 403–412.
Saris CGJ, Horvath S, van Vught PWJ, van Es MA, Blauw HM, Fuller TF et al. Weighted gene co-expression network analysis of the peripheral blood from Amyotrophic Lateral Sclerosis patients. BMC Genomics 2009; 10: 405.
Alexson TO, Hitoshi S, Coles BL, Bernstein A, van der Kooy D . Notch signaling is required to maintain all neural stem cell populations - Irrespective of spatial or temporal niche. Dev Neurosci 2006; 28: 34–48.
Breunig JJ, Silbereis J, Vaccarino FM, Sestan N, Rakic P . Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc Natl Acad Sci USA 2007; 104: 20558–20563.
Lathia JD, Mattson MP, Cheng A . Notch: from neural development to neurological disorders. J Neurochem 2008; 107: 1471–1481.
Huang DW, Sherman BT, Lempicki RA . Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009; 4: 44–57.
Lips ES, Cornelisse LN, Toonen RF, Min JL, Hultman CM, Consortium tIS et al. Functional gene group analysis identifies synaptic gene groups as risk factor for schizophrenia. Mol Psychiatry 2011; 17: 996–1006.
West AK, Hidalgo J, Eddins D, Levin ED, Aschner M . Metallothionein in the central nervous system: roles in protection, regeneration and cognition. Neurotoxicology 2008; 29: 489–503.
Choi KH, Elashoff M, Higgs BW, Song J, Kim S, Sabunciyan S et al. Putative psychosis genes in the prefrontal cortex: combined analysis of gene expression microarrays. BMC Psychiatry 2008; 8: 87.
Weinberger DR . Implications of normal brain-development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 1987; 44: 660–669.
Pantelis C, Yucel M, Wood SJ, Velakoulis D, Sun DQ, Berger G et al. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull 2005; 31: 672–696.
Myers RA, Casals F, Gauthier J, Hamdan FF, Keebler J, Boyko AR et al. A population genetic approach to mapping neurological disorder genes using deep resequencing. PLoS Genet 2011; 7: e1001318.
Shao L, Vawter MP . Shared gene expression alterations in schizophrenia and bipolar disorder. Biol Psychiatry 2008; 64: 89–97.
Jia P, Wang L, Fanous AH, Pato CN, Edwards TL et alThe Internatio nal Schizophrenia Consortium. Network-assisted investigation of combined causal signals from genome-wide association studies in schizophrenia. PLoS Comput Biol 2012; 8: e1002587.
Craddock N, O'Donovan MC, Owen MJ . Genes for schizophrenia and bipolar disorder? Implications for psychiatric nosology. Schizophr Bull 2006; 32: 9–16.
Acknowledgements
This work was funded by 5R01MH080425 (to C.L.), and supported by the Geraldi Norton Foundation and the Eklund Family. We thank Elizabeth Thomas and Ali Torkamani for sharing Victorian Brain Bank Network (VBBN) brain expression data with us. We thank investigators of Wellcome Trust Case–Control Consortium (WTCCC) for sharing non-psychiatric disease type 2 diabetes GWAS data. We thank the investigators of Bipolar Genome Study (BiGS) for generating those bipolar GAIN-BD and Tgen-BD GWAS data, thank investigators of GAIN for generating SZ GWAS data. We thank Seth E. Dobrin, Maree Webster and other collaborators at the Stanley Medical Research Institute for providing the Stanley Online Genomics Database and brain samples. We also thank Lorenzo Pesce, Alex Rodriguez and collaborators at Computation Institute, University of Chicago for supplying their supercomputing devices and services.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, C., Cheng, L., Grennan, K. et al. Two gene co-expression modules differentiate psychotics and controls. Mol Psychiatry 18, 1308–1314 (2013). https://doi.org/10.1038/mp.2012.146
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2012.146
Keywords
This article is cited by
-
Brain and blood transcriptome profiles delineate common genetic pathways across suicidal ideation and suicide
Molecular Psychiatry (2024)
-
Molecular mapping of a core transcriptional signature of microglia-specific genes in schizophrenia
Translational Psychiatry (2023)
-
Differences of resting fMRI and cognitive function between drug-naïve bipolar disorder and schizophrenia
BMC Psychiatry (2022)
-
Evaluation of animal model congruence to human depression based on large-scale gene expression patterns of the CNS
Scientific Reports (2022)
-
Rare variants implicate NMDA receptor signaling and cerebellar gene networks in risk for bipolar disorder
Molecular Psychiatry (2022)