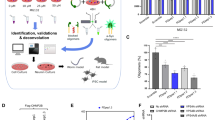
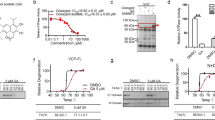
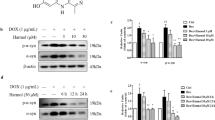
Abstract
Latrepirdine (Dimebon; dimebolin) is a neuroactive compound that was associated with enhanced cognition, neuroprotection and neurogenesis in laboratory animals, and has entered phase II clinical trials for both Alzheimer's disease and Huntington's disease (HD). Based on recent indications that latrepirdine protects cells against cytotoxicity associated with expression of aggregatable neurodegeneration-related proteins, including Aβ42 and γ-synuclein, we sought to determine whether latrepirdine offers protection to Saccharomyces cerevisiae. We utilized separate and parallel expression in yeast of several neurodegeneration-related proteins, including α-synuclein (α-syn), the amyotrophic lateral sclerosis-associated genes TDP43 and FUS, and the HD-associated protein huntingtin with a 103 copy-polyglutamine expansion (HTT gene; htt-103Q). Latrepirdine effects on α-syn clearance and toxicity were also measured following treatment of SH-SY5Y cells or chronic treatment of wild-type mice. Latrepirdine only protected yeast against the cytotoxicity associated with α-syn, and this appeared to occur via induction of autophagy. We further report that latrepirdine stimulated the degradation of α-syn in differentiated SH-SY5Y neurons, and in mouse brain following chronic administration, in parallel with elevation of the levels of markers of autophagic activity. Ongoing experiments will determine the utility of latrepirdine to abrogate α-syn accumulation in transgenic mouse models of α-syn neuropathology. We propose that latrepirdine may represent a novel scaffold for discovery of robust pro-autophagic/anti-neurodegeneration compounds, which might yield clinical benefit for synucleinopathies including Parkinson's disease, Lewy body dementia, rapid eye movement (REM) sleep disorder and/or multiple system atrophy, following optimization of its pro-autophagic and pro-neurogenic activities.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lermontova NN, Lukoyanov NV, Serkova TP, Lukoyanova EA, Bachurin SO . Dimebon improves learning in animals with experimental Alzheimer's disease. Bull Exp Biol Med 2000; 129: 544–546.
Grigorev VV, Dranyi OA, Bachurin SO . Comparative study of action mechanisms of dimebon and memantine on AMPA- and NMDA-subtypes glutamate receptors in rat cerebral neurons. Bull Exp Biol Med 2003; 136: 474–477.
Giorgetti M, Gibbons JA, Bernales S, Alfaro IE, Drieu La Rochelle C, Cremers T et al. Cognition-enhancing properties of dimebon in a rat novel object recognition task are unlikely to be associated with acetylcholinesterase inhibition or N-methyl-D-aspartate receptor antagonism. J Pharmacol Exp Ther 2010; 333: 748–757.
Wang J, Ferruzzi MG, Varghese M, Qian X, Cheng A, Xie M et al. Preclinical study of dimebon on beta-amyloid-mediated neuropathology in Alzheimer's disease. Mol Neurodegener 2011; 6: 7.
Bachurin S, Bukatina E, Lermontova N, Tkachenko S, Afanasiev A, Grigoriev V et al. Antihistamine agent dimebon as a novel neuroprotector and a cognition enhancer. Ann NY Acad Sci 2001; 939: 425–435.
Wu J, Li Q, Bezprozvanny I . Evaluation of dimebon in cellular model of Huntington's disease. Mol Neurodegener 2008; 3: 15.
Pieper AA, Xie S, Capota E, Estill SJ, Zhong J, Long JM et al. Discovery of a prone urogenic, neuroprotective chemical. Cell 2010; 142: 39–51.
Doody RS, Gavrilova SI, Sano M, Thomas RG, Aisen PS, Bachurin SO et al. Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer's disease: a randomised, double-blind, placebo-controlled study. Lancet 2008; 372: 207–215.
Kieburtz K, McDermott MP, Voss TS, Corey-Bloom J, Deuel LM, Dorsey ER et al. A randomized, placebo-controlled trial of latrepirdine in Huntington disease. Arch Neurol 2010; 67: 154–160.
Bharadwaj P, Verdile G, Barr RK, Gupta V, Steele JW, Lachenmayer ML et al. Latrepirdine (Dimebon™) enhances autophagy and reduces intracellular GFP-Aβ42 levels in yeast. J Alzheimer's Disease (in press).
Bachurin SO, Shelkovnikova TA, Ustyugov AA, Peters O, Khritankova I, Afanasieva MA et al. Dimebon slows progression of proteinopathy in gamma-synuclein transgenic mice. Neurotox Res 2012; 22: 33–42.
Walsh DM, Selkoe DJ . Oligomers on the brain: the emerging role of soluble protein aggregates in neurodegeneration. Protein Peptide Lett 2004; 11: 213–228.
Broe M, Shepherd CE, Mann DM, Milward EA, Gai WP, Thiel E et al. Insoluble alpha-synuclein in Alzheimer's disease without Lewy body formation. Neurotox Res 2005; 7: 69–76.
Uchikado H, Lin WL, DeLucia MW, Dickson DW . Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-synucleinopathy. J Neuropathol Exp Neurol 2006; 65: 685–697.
Wirths O, Bayer TA . Alpha-synuclein, Abeta and Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry 2003; 27: 103–108.
Bartels T, Choi JG, Selkoe DJ . Alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 2011; 477: 107–110.
Tian Y, Bustos V, Flajolet M, Greengard P . A small-molecule enhancer of autophagy decreases levels of Abeta and APP-CTF via Atg5-dependent autophagy pathway. FASEB J 2011; 25: 1934–1942.
Sarkar S, Perlstein EO, Imarisio S, Pineau S, Cordenier A, Maglathlin RL et al. Small molecules enhance autophagy and reduce toxicity in Huntington's disease models. Nat Chem Biol 2007; 3: 331–338.
Steele JW, Lachenmayer ML, Ju S, Stock A et al. Latrepirdine improves cognition and arrests progression of neuropathology in an Alzheimer's mouse model. submitted manuscript.
Steele JW, Kim SH, Cirrito JR, Verges DK, Restivo JL, Westaway D et al. Acute dosing of latrepirdine (Dimebon), a possible Alzheimer therapeutic, elevates extracellular amyloid-beta levels in vitro and in vivo. Mol Neurodegener 2009; 4: 51.
Klionsky DJ . Monitoring autophagy in yeast: the Pho8Delta60 assay. Methods Mol Biol 2007; 390: 363–371.
Vekrellis K, Xilouri M, Emmanouilidou E, Stefanis L . Inducible over-expression of wild type alpha-synuclein in human neuronal cells leads to caspase-dependent non-apoptotic death. J Neurochem 2009; 109: 1348–1362.
Outeiro TF, Lindquist S . Yeast cells provide insight into alpha-synuclein biology and pathobiology. Science 2003; 302: 1772–1775.
Tanaka M, Chien P, Yonekura K, Weissman JS . Mechanism of cross-species prion transmission: an infectious conformation compatible with two highly divergent yeast prion proteins. Cell 2005; 121: 49–62.
Noda T, Ohsumi Y . Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J Biol Chem 1998; 273: 3963–3966.
Yue Z, Friedman L, Komatsu M, Tanaka K . The cellular pathways of neuronal autophagy and their implication in neurodegenerative diseases. Biochim Biophys Acta 2009; 1793: 1496–1507.
Knauer MF, Soreghan B, Burdick D, Kosmoski J, Glabe CG . Intracellular accumulation and resistance to degradation of the Alzheimer amyloid A4/beta protein. Proc Natl Acad Sci USA 1992; 89: 7437–7441.
Ebrahimi-Fakhari D, Cantuti-Castelvetri I, Fan Z, Rockenstein E, Masliah E, Hyman BT et al. Distinct roles in vivo for the ubiquitin-proteasome system and the autophagy-lysosomal pathway in the degradation of alpha-synuclein. J Neurosci 2011; 31: 14508–14520.
Friedman LG, Lachenmayer ML, Wang J, He L, Poulose SM, Komatsu M et al. Disrupted autophagy leads to dopaminergic axon and dendrite degeneration and promotes presynaptic accumulation of α-synuclein and LRRK2 in the brain. J Neurosci 2012; 32: 7585–7593.
Miller G . Pharmacology. The puzzling rise and fall of a dark-horse Alzheimer's drug. Science 2010; 327: 1309.
Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science 2006; 313: 324–328.
Ju S, Tardiff DF, Han H, Divya K, Zhong Q, Maquat LE et al. A yeast model of FUS/TLS-dependent cytotoxicity. PLoS Biol 2011; 9: e1001052.
Johnson BS, McCaffery JM, Lindquist S, Gitler AD . A yeast TDP-43 proteinopathy model: exploring the molecular determinants of TDP-43 aggregation and cellular toxicity. Proc Natl Acad Sci USA 2008; 105: 6439–6444.
Willingham S, Outeiro TF, DeVit MJ, Lindquist SL, Muchowski PJ . Yeast genes that enhance the toxicity of a mutant huntingtin fragment or alpha-synuclein. Science 2003; 302: 1769–1772.
He C, Song H, Yorimitsu T, Monastyrska I, Yen WL, Legakis JE et al. Recruitment of Atg9 to the preautophagosomal structure by Atg11 is essential for selective autophagy in budding yeast. J Cell Biol 2006; 175: 925–935.
Acknowledgements
The work in this manuscript was used in a dissertation by JWS as partial requirement for the fulfillment of the PhD degree. JWS is a trainee in the Integrated Pharmacological Sciences Training Program supported by grant T32GM062754 from the National Institute of General Medical Sciences. MLL was supported by the Deutsche Forschungsgemeinschaft. SG is a member of the Oligomer Research Consortium of the Cure Alzheimer's Fund. We acknowledge the generous support of the NH&MRC (APP1009295 to RM, GV, SG), McCusker Alzheimer's Research Foundation (RM, GV), Conicyt (PFB-16 to SB), Fidelity Biosciences Research Initiative (SJ, JL, DR, GAP), Cure Alzheimer's Fund (SG), the US Department of Veterans Affairs (SG), and the NIH (P01AG10491 to SG; P50AG05138 to Mary Sano; P30 NS061777 and S10 RR022415 to RW; R01NS060123 and U54RR022220 to ZY). We would like to thank Rosilyn Kazanjian for her gift in memory of Powel Kazanjian. We would also like to thank Loren E Khan and Justine Bonet for technical assistance in animal colony management and Dr Yun Zhong for technical support, and Dr Kostas Vekrellis (Foundation for Biomedical Research of the Academy of Athens, Athens, Greece) for generously providing the tet-off inducible α-syn overexpressing SH-SY5Y neuroblastoma cell line.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
AAP is Vice President of Preclinical Development for Medivation. SG holds research grant support from Amicus Pharmaceuticals and is a consultant to the Pfizer-Janssen Alzheimer's Immunotherapy Alliance. GAP is on the scientific advisory boards of Amicus Pharmaceuticals and Neurophage. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Steele, J., Ju, S., Lachenmayer, M. et al. Latrepirdine stimulates autophagy and reduces accumulation of α-synuclein in cells and in mouse brain. Mol Psychiatry 18, 882–888 (2013). https://doi.org/10.1038/mp.2012.115
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2012.115
Keywords
This article is cited by
-
Role of lysosomes in physiological activities, diseases, and therapy
Journal of Hematology & Oncology (2021)
-
Autophagy-dependent removal of α-synuclein: a novel mechanism of GM1 ganglioside neuroprotection against Parkinson’s disease
Acta Pharmacologica Sinica (2021)
-
Pro-neurogenic, Memory-Enhancing and Anti-stress Effects of DF302, a Novel Fluorine Gamma-Carboline Derivative with Multi-target Mechanism of Action
Molecular Neurobiology (2018)
-
Therapeutic potential of autophagy-enhancing agents in Parkinson’s disease
Molecular Neurodegeneration (2017)
-
Lysosomal dysfunction in the brain of a mouse model with intraneuronal accumulation of carboxyl terminal fragments of the amyloid precursor protein
Molecular Psychiatry (2017)