Abstract
Erythroleukemia was considered an acute myeloid leukemia in the 2008 World Health Organization (WHO) classification and is defined by the presence of ≥50% bone marrow erythroblasts, having <20% bone marrow blasts from total nucleated cells but ≥20% bone marrow myeloblasts from nonerythroid cells. Erythroleukemia shares clinicopathologic features with myelodysplastic syndromes, especially with erythroid-predominant myelodysplastic syndromes (≥50% bone marrow erythroblasts). The upcoming WHO revision proposes to eliminate the nonerythroid blast cell count rule and to move erythroleukemia patients into the appropriate myelodysplastic syndrome category on the basis of the absolute blast cell count. We conducted a retrospective study of patients with de novo erythroleukemia and compared their clinico-biological features and outcome with those of de novo myelodysplastic syndromes, focusing on erythroid-predominant myelodysplastic syndromes. Median overall survival of 405 erythroid-predominant myelodysplastic syndromes without excess blasts was significantly longer than that observed in 57 erythroid-predominant refractory anemias with excess blasts-1 and in 59 erythroleukemias, but no significant difference was observed between erythroid-predominant refractory anemias with excess blasts-1 and erythroleukemias. In this subset of patients with ≥50% bone marrow erythroblasts and excess blasts, the presence of a high-risk karyotype defined by the International Prognostic Scoring System or by the Revised International Prognostic Scoring System was the main prognostic factor. In the same way, the survival of 459 refractory anemias with excess blasts-2, independently of having ≥20% bone marrow blasts from nonerythroid cells or not, was almost identical to the observed in 59 erythroleukemias. Interestingly, 11 low-blast count erythroleukemias with 5 to <10% bone marrow blasts from total nucleated cells showed similar survival than the rest of erythroleukemias. Our data suggest that de novo erythroleukemia is in the spectrum of myelodysplastic syndromes with excess blasts and support its inclusion into future classifications of myelodysplastic syndromes.
Similar content being viewed by others
Main
Acute erythroid leukemias are characterized by a predominant erythroid population and represent less than 5% of cases of adult acute myeloid leukemias. The 2008 World Health Organization (WHO) recognizes two subtypes of acute erythroid leukemia based on the presence or absence of a significant myeloblastic component: erythroleukemia and pure erythroid leukemia. Erythroleukemia (erythroid/myeloid) is defined by the presence of equal or greater than 50% erythroid precursors in bone marrow and equal or greater than 20% myeloblasts in nonerythroid cells. Pure erythroid leukemia represents a neoplastic proliferation of erythroid precursors (equal or greater than 80% of bone marrow cells) with no evidence of a significant myeloblastic component.1, 2
Erythroleukemia shares clinicopathologic features with myelodysplastic syndromes: multilineage dysplasia is common, presents a high rate of myelodysplastic syndrome-like cytogenetic abnormalities and shows a mutation profile that is closer to myelodysplastic syndromes than to de novo acute myeloid leukemia.3, 4, 5, 6, 7, 8, 9, 10 Those cases with equal or greater than 50% bone marrow erythroblasts but not fulfilling criteria for erythroleukemia due to a percentage of bone marrow myeloblasts lower than 20% from nonerythroid cells, are considered erythroid-predominant myelodysplastic syndromes and, following current WHO recommendation, their bone marrow blast percentage must be considered from total bone marrow nucleated cells.2, 11, 12, 13 Possible diagnoses when erythroid precursors are equal or greater than 50% of bone marrow nucleated cells are summarized in Supplementary Table S1.
Interestingly, as a flaw of current WHO recommendation, refractory anemia with excess blasts-2 diagnosis is extremely rare in myelodysplastic syndromes with erythroid predominance, as patients with the minimum percentage of bone marrow blasts to meet diagnosis criteria for refractory anemia with excess blasts-2 (equal or greater than 10% of bone marrow blasts from total nucleated cells) having ≥50% bone marrow erythroblasts would present always at least 20% bone marrow blasts from nonerythroid cells, fulfilling erythroleukemia criteria. Therefore, refractory anemia with excess blasts-1 is almost invariably the highest WHO risk category applicable in this subset of patients. The uncommon situation where refractory anemia with excess blasts-2 diagnosis could be established in erythroid-predominant myelodysplastic syndromes are those cases with less than 10% bone marrow blasts from total nucleated cells but with 5% to less than 20% blasts in peripheral blood or when Auer rods are present. Hence, erythroleukemia would really represent the next-highest risk category of erythroid-predominant refractory anemia with excess blasts-1 if the former were considered a specific subtype of erythroid-predominant myelodysplastic syndrome. However, there are no data in the literature about a specific comparison between these two entities.
Few studies have compared clinical features and outcome of de novo erythroleukemia with those observed in erythroid-rich myelodysplastic syndromes. Furthermore, most of them also collected cases of therapy-related myeloid neoplasms and secondary erythroleukemias.6, 8, 9, 10
A revision of the fourth edition of WHO Classification of myelodysplastic syndromes, originally published in 2008, is expected this year. As mentioned in a recent publication by Arber et al14, 15 that summarizes major changes in the upcoming WHO classification, the nonerythroid blast cell count rule will be eliminated and those patients that fulfilled erythroleukemia criteria will be considered myelodysplastic syndromes and will be moved into the appropriate myelodysplastic syndrome category based on the absolute blast cell count.
For all the above, an extensive comparison between de novo erythroleukemia and myelodysplastic syndromes, especially myelodysplastic syndromes with erythroid predominance, seems crucial to elucidate whether these entities should really be considered as a continuum instead of two different biological entities.
Materials and methods
Patients
From 1972 to 2015, we retrospectively collected clinical and laboratory data of 3687 de novo myelodysplastic syndromes included in the Spanish Registry of Myelodysplastic Syndromes, 462 of whom were erythroid-predominant myelodysplastic syndromes (42 refractory cytopenias with unilineage dysplasia, 139 refractory anemias with ring sideroblasts, 200 refractory cytopenias with multilineage dysplasia, 12 myelodysplastic syndromes with isolated del(5q), 12 myelodysplastic syndromes-unclassified, and 57 refractory anemias with excess blasts-1). We could not establish refractory anemia with excess blasts-2 diagnosis in any erythroid-predominant myelodysplastic syndrome of our series. Moreover, 59 de novo erythroleukemias were collected in the same period. A typical case of erythroleukemia displaying a complex karyotype is shown in Figure 1. Morphology, laboratory characteristics, cytogenetics, and clinical follow-up were available for all the patients. Diagnosis of myelodysplastic syndromes and erythroleukemia was made according to the 2008 WHO classification proposals.2 Those patients diagnosed initially by French–American–British classification criteria,16, 17, 18, 19 were reclassified according to the WHO proposals, based on morphological, laboratory, and cytogenetic data. Information about percentage of bone marrow blasts from total nucleated cells and bone marrow erythroblasts was available in all 3687 de novo myelodysplastic syndrome patients. Further, the bone marrow blast percentage from nonerythroid cells was calculated as follows: [% bone marrow blasts from total nucleated cells/(100−% bone marrow erythroblasts)] × 100. Distribution of patients among WHO categories is depicted in Supplementary Table S2. Patients diagnosed with secondary myelodysplastic syndrome (therapy-related myeloid neoplasm) or secondary erythroleukemia (therapy-related or evolved from primary myelodysplastic syndrome), pure erythroid leukemia, chronic myelomonocytic leukemia, and unclassified myelodysplastic/myeloproliferative neoplasms were excluded from the present study. The study was conducted fulfilling the biomedical research guidelines of Declaration of Helsinki.
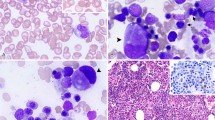
Morphologic features of de novo erythroleukemia. (a) Bone marrow smear shows immature erythroblasts with vacuolated cytoplasm (May–Grünwald–Giemsa). (b) Diffuse pattern in the cytoplasm of mature erythroblasts and granular and clumped pattern in earlier precursors by periodic acid-Schiff reaction (PAS) stain. (c) This patient presented a complex karyotype, a common finding in erythroleukemia: 44–45,XY,−5,−14,−17,+mar,+r[cp10]/44–45,XY,−5,add(10)(p15),−14,-17,+mar,+r[cp3]/44–45,XY,−5,add(10)(p11),−17,add(19)(q13),+mar[cp2]/46,XY[5].
Morphological Studies
At least, two bone marrow and one peripheral blood May–Grünwald–Giemsa-stained smears were used for conducting the morphologic analysis at the individual centers. In addition, a Prussian blue-stained bone marrow smear was used for assessing the percentage of ring sideroblasts. The WHO 2008 proposals for evaluating the morphological diagnosis of myelodysplastic syndrome and erythroleukemia were followed strictly.2 As recommended, peripheral blood and bone marrow differential counts were performed on at least 200 and 500 cells, respectively. Bone marrow blast counts were assessed from total bone marrow nucleated cells. Following the 2008 WHO recommendations, the threshold used for considering a myeloid cell line as dysplastic was the presence of ≥10% abnormal cells in the corresponding myeloid lineage. For the evaluation of dysplasia, at least 200 neutrophils, 200 erythroblasts, and 30 megakaryocytes were assessed in bone marrow. Multilineage dysplasia was defined by dysplasia involving two or more lineages. As currently recommended by the Spanish Guidelines for the diagnosis and treatment of myelodysplastic syndromes and chronic myelomonocytic leukemia,20 bone marrow biopsy was conducted only in those cases where fibrosis, hypoplastic myelodysplastic syndromes, or idiophatic cytopenias of undetermined significance were suspected.
Conventional Cytogenetic Studies
Cytogenetic analyses were performed on G-banded chromosomes obtained from 24 h unstimulated bone marrow cultures at the individual centers. When possible, at least 20 metaphases per sample were studied. Karyotypes were described according to the International System for Human Cytogenetic Nomenclature.21
Statistical Analysis
Statistical analyses were performed using the statistical package for the social sciences software version 22.0 (SPSS, Chicago, IL, USA). Categorical variables were described by frequencies and percentages; and continuous variables by medians and ranges. The assumption of normality was assessed by using the Shapiro–Wilk test. For categorical data, comparisons of proportions were evaluated by Chi-square test. For continuous variables, comparisons of medians, ranks and means were assessed by nonparametric (Mood’s median or Mann–Whitney U-tests) or parametric tests (t-test) as appropriate according to the distribution of the studied variables. Mood’s median test was selected instead of Mann–Whitney U-test, as a more robust method to compare those variables showing outlier values. Survival curves were constructed by Kaplan–Meier method using the interval from the date of diagnosis to the date of last contact or death (overall survival) and compared using the log-rank test. Patients undergoing hematopoietic allogeneic stem cell transplantation or intensive acute myeloid leukemia-type chemotherapy were censored at the date of starting chemotherapy or the date of transplant, whichever came first, except when the prognostic impact of allogeneic stem cell transplantation was assessed. Differences were considered statistically significant when P-values were <0.05 in a two-tailed test. Multivariable analysis was performed using Cox’s proportional hazards regression model to assess the independent prognostic impact of variables that showed significance in the univariable overall survival analysis. The assumption of proportional hazards over time was tested for all explanatory covariates by using a time-dependent covariate.
Results
Clinical follow-up data were available for all the patients. The estimated median follow-up in the overall series, as calculated by the reverse Kaplan–Meier method was 42 months, 44 months, and 42 months for myelodysplastic syndromes with or without erythroid predominance, respectively. At the last follow-up, 1642 of 3687 patients (44.5%) had died. The estimated median follow-up for de novo erythroleukemia patients, as calculated by the reverse Kaplan–Meier method was 30 months. At the last follow-up, 43 of 59 patients (72.9%) had died.
Erythroid-Predominant Refractory Anemia with Excess Blasts-1 and De novo Erythroleukemia Share Clinico-Biological Features and Outcome
Refractory anemia with excess blasts-1 was the highest WHO risk category applicable in erythroid-predominant myelodysplastic syndromes of our series, since patients with erythroid-predominant myelodysplastic syndrome with equal or greater than 10% bone marrow blasts from total nucleated cells, which would be classified as refractory anemia with excess blasts-2 if bone marrow erythroblasts were less than 50%, fulfilled erythroleukemia criteria. Thus, we wanted to compare clinical features, cytogenetics, and outcome of erythroid-predominant refractory anemia with excess blasts-1 with those of erythroleukemia, as this would represent the next-highest risk category if this were considered a specific subtype of myelodysplastic syndrome. Comparison of the main clinico-biological features between 59 de novo erythroleukemias and 57 erythroid-predominant refractory anemias with excess blasts-1 is depicted in Table 1. Interestingly, no significant differences were observed according to age, sex distribution, hemoglobin levels, neutrophil count, median percentage of bone marrow erythroblasts, median percentage of dysplasia of the different myeloid series, and the International Prognostic Scoring System22 or Revised International Prognostic Scoring System23 cytogenetic risk category distribution. Moreover, no significant differences were observed in the percentage of high-risk karyotypes defined by the International Prognostic Scoring System or by the Revised International Prognostic Scoring System (poor and very poor categories) between erythroid-predominant refractory anemia with excess blasts-1 and erythroleukemia (International Prognostic Scoring System, 28 vs 19%, P=0.230; Revised International Prognostic Scoring System, 28 vs 22%, P=0.453). Very few differences were observed: platelet count was significantly inferior in erythroleukemia and, as expected due to current definition of these entities, bone marrow blast percentage considered from total nucleated cells or from nonerythroid cells was significantly inferior in erythroid-predominant refractory anemia with excess blasts-1. No significant difference was observed between erythroleukemia and erythroid-predominant refractory anemia with excess blasts-1 in the proportion of patients receiving acute myeloid leukemia-type chemotherapy (18.6 vs 12.3%, P=0.344), allogeneic stem cell transplantation (18.6 vs 8.8%, P=0.123) or at least one of them (27.1 vs 17.5%, P=0.216). Azacitidine was approved in Spain in January 2009 for the treatment of higher-risk myelodysplastic syndromes and acute myeloid leukemia with 20–30% bone marrow blasts from total nucleated cells (previously considered refractory anemia with excess blasts in transformation). Although azacitidine is not currently approved for the treatment of erythroleukemia, its use is widely extended in this elderly group of patients usually unfit to receive common acute myeloid leukemia-type treatments or not eligible for allogeneic stem cell transplantation. On assessing the patients diagnosed from 2009 onward, a higher trend was observed in the proportion of erythroleukemias receiving azacitidine compared with erythroid-predominant refractory anemias with excess blasts-1, although no significant difference was detected (53.3 vs 29.2%, P=0.074).
Although overall survival of 405 erythroid-predominant myelodysplastic syndromes without excess of blasts (<5% bone marrow blasts from total nucleated cells) was significantly longer than that observed in 57 erythroid-predominant refractory anemias with excess blasts-1 (5 to <10% bone marrow blasts from total nucleated cells) and in 59 de novo erythroleukemias (10 to <20% bone marrow blasts from total nucleated cells) (median overall survival, 71 vs 20 months, P<0.001; median overall survival, 71 vs 13 months, P<0.001; respectively), no significant difference in the overall survival was observed between 57 erythroid-predominant refractory anemias with excess blasts-1 and 59 de novo erythroleukemias (median overall survival, 20 vs 13 months; P=0.33; Figure 2). Interestingly, erythroid-predominant refractory anemia with excess blasts-1 patients showed a significant shorter overall survival than 489 refractory anemia with excess blasts-1 with less than 50% bone marrow erythroblasts (median overall survival, 20 vs 34 months; P=0.042; Supplementary Figure S1). This could be explained, at least in part, by the difference in the median percentage of bone marrow blasts calculated from nonerythroid cells. Although the median percentage of bone marrow blasts from total nucleated cells was higher in refractory anemia with excess blasts-1 with less than 50% bone marrow erythroblasts than in erythroid-predominant refractory anemia with excess blasts-1 (6.8 vs 6%, P=0.044), erythroid-predominant refractory anemia with excess blasts-1 displayed a significant higher median percentage of bone marrow blasts from nonerythroid cells (15 vs 9.38%, P<0.001). In the same way, erythroleukemia patients showed a significant shorter overall survival than 489 refractory anemia with excess blasts-1 with less than 50% bone marrow erythroblasts (median overall survival, 13 vs 34 months; P=0.001).
Overall survival by Kaplan–Meier analysis of 57 refractory anemias with excess blasts-1 with ≥50% bone marrow erythroblasts (RAEB1-E) vs 59 de novo erythroleukemias. No significant difference in overall survival was observed (median overall survival, 20 vs 13 months; NS). Overall survival is indicated in months and was compared with the two-sided log-rank test.
Changes in the different standards applied to diagnosis and management of patients over time could exercise an influence on the survival analysis, especially on series collected during long time. To assess this potential bias in our study, we divided our series in three time periods: 1972 to <2000, 2000 to <2009, and 2009 to present. These thresholds were selected attending to the increased indication of allogeneic stem cell transplantation in the 2000 to <2009 era and the use of azacitidine as a standard of care in Spain for higher-risk myelodysplastic syndromes since 2009. No significant difference was observed in the proportion of patients diagnosed with de novo erythroleukemia or with erythroid-predominant refractory anemia with excess blasts-1 in each one of these periods (15.3% of erythroleukemias vs 19.3% of erythroid-predominant refractory anemias with excess blasts-1 in the first period, 33.9% of erythroleukemias vs 38.6% of erythroid-predominant refractory anemias with excess blasts-1 in the second period, 50.8% of erythroleukemias vs 42.1% of erythroid-predominant refractory anemias with excess blasts-1 in the third period; P=0.629).
The low incidence of these diseases makes difficult to perform studies with larger series. Nevertheless, our findings evidence that de novo erythroleukemia shares clinico-biological features and outcome with erythroid-predominant refractory anemia with excess blasts-1.
Then, we assessed International Prognostic Scoring System and Revised International Prognostic Scoring System cytogenetic risk classification in the subset of 116 patients with erythroid predominance and excess of blasts (erythroleukemia and erythroid-predominant refractory anemia with excess blasts-1). The presence of a high-risk karyotype according to both, International Prognostic Scoring System and Revised International Prognostic Scoring System, was capable to discriminate two risk groups with significant different overall survival (median overall survival, 20 vs 10 months, P=0.001; median overall survival, 20 vs 10 months, P=0.001; respectively; Figure 3). Moreover, other classical myelodysplastic syndrome prognostic factors were assessed and, surprisingly, only hemoglobin <100 g/l showed an impact (median overall survival, 36 vs 11 months; P=0.006). Platelets <100 × 109/l, platelets <50 × 109/l, neutrophils <500/mm3, neutrophils <800/mm3 showed no impact in terms of overall survival. Furthermore, the 10% bone marrow blast cutoff segregates a group of 48 de novo erythroleukemias that showed no significant differences in overall survival compared with the rest of the patients with erythroid predominance and excess of blasts (11 erythroleukemias with less than 10% bone marrow blasts from total nucleated cells and 57 erythroid-predominant refractory anemias with excess blasts-1; median overall survival, 13 vs 18 months; P=0.61). In the same way, no significant difference in overall survival was observed between the 11 low-blast count erythroleukemias with less than 10% bone marrow blasts from total nucleated cells and the rest of erythroleukemias (median overall survival, 13 vs 13 months, P=0.518). Moreover, the survival of these low-count erythroleukemias was similar to that observed in all 459 refractory anemia with excess blasts-2 (median overall survival, 13 vs 14 months, P=0.916).
Overall survival by Kaplan–Meier analysis. (a) Survival according to the presence of an International Prognostic Scoring System (IPSS) high-risk karyotype or not (median overall survival, 10 vs 20 months, P=0.001). (b) Survival according to the presence of a Revised International Prognostic Scoring System (IPSS-R) high-risk karyotype (poor and very poor) or not (median overall survival, 10 vs 20 months, P=0.001). Overall survival is indicated in months and was compared with the two-sided log-rank test.
Afterwards, the prognostic impact of allogeneic stem cell transplantation in this subset of 116 patients with erythroid predominance and excess of blasts was assessed. The 16 patients who underwent an allogeneic stem cell transplantation showed a significant longer overall survival than those who did not (median overall survival, NR vs 17 months; P=0.017).
Finally, we carried out a multivariable analysis of overall survival by using Cox regression modeling including those variables that showed an impact on overall survival in the univariable survival analysis: age, high-risk International Prognostic Scoring System cytogenetic category, hemoglobin <100 g/l, and allogeneic stem cell transplantation as a time-dependent variable. Only the high-risk International Prognostic Scoring System cytogenetic category retained its prognostic influence on overall survival (Table 2a). Further, when the Cox regression model was carried out introducing the high-risk Revised International Prognostic Scoring System cytogenetic group (poor and very poor categories) instead of the high-risk International Prognostic Scoring System cytogenetic category, again, only the high-risk Revised International Prognostic Scoring System cytogenetic group retained its independent prognostic influence on overall survival (Table 2b).
Refractory Anemia with Excess Blasts-2 and De novo Erythroleukemia Showed a Similar Outcome
We compared the outcome of 59 de novo erythroleukemias with that of 459 patients diagnosed with refractory anemia with excess blasts-2 and no significant difference in overall survival was observed (median overall survival, 13 vs 14 months; P=0.65; Figure 4a). Then, survival of erythroleukemia and myelodysplastic syndromes with less than 50% bone marrow erythroblasts that, as in erythroleukemia, presented equal or greater than 20% bone marrow blasts from nonerythroid cells was also compared. We detected 175 of 459 refractory anemias with excess blasts-2 (38%) meeting this requirement. These two groups of patients presented a similar overall survival (median overall survival, 13 vs 13 months; P=0.72; Figure 4b). This finding underlines the arbitrariness of the established erythroblast cutoff to consider the diagnosis of erythroleukemia and the absence of prognostic impact per se of the erythroid predominance.
Overall survival by Kaplan–Meier analysis of (a) 59 de novo erythroleukemias vs 459 refractory anemias with excess blasts-2 (RAEB-2) (median overall survival, 13 vs 14 months; NS) and (b) 59 de novo erythroleukemias vs 175 refractory anemias with excess blasts-2 with equal or greater than 20% bone marrow blasts from nonerythroid cells (NECs) (median overall survival, 13 vs 13 months; NS). Overall survival is indicated in months and was compared with the two-sided log-rank test.
Afterwards, a comparison of the main clinico-biological features of de novo erythroleukemia and refractory anemia with excess blasts-2 was carried out and only significant differences in median percentage of bone marrow erythroblasts, median percentage of dysgranulopoiesis, and median percentage of bone marrow blasts from nonerythroid cells were detected. Interestingly, no significant differences were observed according to International Prognostic Scoring System or Revised International Prognostic Scoring System cytogenetic risk category distribution, median percentage of dysplasia of the erythroid and megakaryocytic lineages, median percentage of bone marrow blasts from total nucleated cells, hemoglobin levels, neutrophil count, platelet count, age and sex distribution. All these comparisons are depicted in Table 3. Once again, these results point out that erythroleukemia is in the spectrum of myelodysplastic syndromes with excess blasts.
Finally, no significant difference was observed between erythroleukemia and refractory anemia with excess blasts-2 in the proportion of patients receiving acute myeloid leukemia-type chemotherapy (18.6 vs 17.4%, P=0.817), but a significant higher percentage of erythroleukemia patients underwent an allogeneic stem cell transplantation (18.6 vs 9.6%, P=0.034). Interestingly, when assessing patients diagnosed from 2009 onward, no significant difference was detected in the proportion of patients treated with azacitidine (53.3 vs 55%, P=0.863).
As previously mentioned, to elucidate whether there was a bias on the survival analysis related to differences between groups in diagnosis dates, we compared the proportion of patients diagnosed with erythroleukemia with that diagnosed with refractory anemia with excess blasts-2 in each one of the periods anteriorly proposed (1972 to <2000, 2000 to <2009, and 2009 to present) and no difference was observed (15.3% of erythroleukemias vs 16.1% of refractory anemias with excess blasts-2 in the first period, 33.9% of erythroleukemias vs 35.9% of refractory anemias with excess blasts-2 in the second period, 50.8% of erythroleukemias vs 47.9% of refractory anemias with excess blasts-2 in the third period; P=0.915).
Discussion
Myelodysplastic syndromes and acute myeloid leukemias with equal or greater than 50% erythroblasts in bone marrow represent up to 15% of myelodysplastic syndromes and around 5% of acute myeloid leukemias, respectively.11 In the present study, the largest series of de novo erythroid-predominant myelodysplastic syndromes reported to date and an extensive group of de novo erythroleukemia defined by the WHO 2008 criteria were compared. Clinical, morphological, cytogenetic and molecular features of erythroleukemia have been described in several retrospective studies but most of those articles also collected cases of therapy-related myeloid neoplasms and secondary erythroleukemias.6, 7, 8, 9, 10
Classification of myeloid malignances with expanded erythropoiesis is often difficult and requires integration of clinical, morphological, and cytogenetic features. The definition and classification of erythroleukemia have been changed over the years.1, 2, 16, 17, 18, 19 The 2008 WHO classification recommended to enumerate bone marrow blasts from nonerythroid cells when bone marrow erythroblasts were equal or greater than 50%. In those instances, the disorder was classified as erythroleukemia when bone marrow blasts were equal or greater than 20% and as erythroid-predominant myelodysplastic syndrome when lower. In the latter, percentage of blasts must be considered from total nucleated cells.2 A revision of that classification is expected this year and, as Arber et al mentioned in a recent paper, the elimination of the nonerythroid blast cell count rule will be recommended. Therefore, patients fulfilling erythroleukemia criteria will be now considered myelodysplastic syndromes and will be moved into the appropriate myelodysplastic syndrome category based on the blast cell count from total nucleated cells. Following this recommendation, the majority of patients will be reclassified as myelodysplastic syndromes with excess blasts-2, previously named refractory anemia with excess blasts-2, and a minority of them will be moved to categories with less than 10% bone marrow blasts, mostly myelodysplastic syndromes with excess blasts-1, prior refractory anemia with excess blasts-1.14, 15
Although in the 2008 WHO classification erythroleukemia was included into acute myeloid leukemias,2 our results strongly support the future WHO recommendation of considering erythroleukemia as a myelodysplastic syndrome,14, 15 but we have some concerns related to the disappearance of erythroleukemia as an entity. As it has been shown in our study, erythroleukemia usually presents multilineage dysplasia, typical myelodysplastic syndrome cytogenetic aberrations, and an outcome similar to myelodysplastic syndromes with excess blasts. Moreover, recent studies suggest that cases of erythroleukemia have a molecular profile much closer to myelodysplastic syndromes than to acute myeloid leukemia without erythroid predominance.6, 7, 10 Our results showed that erythroid-predominant refractory anemia with excess blasts-1 presented no significant difference in survival compared with de novo erythroleukemia and in this subset of patients, having erythroid predominance and excess blasts, the presence of a high-risk karyotype defined by the International Prognostic Scoring System or by the Revised International Prognostic Scoring System was the main prognostic factor. Survival of de novo erythroleukemia did not significantly differ from that observed in refractory anemia with excess blasts-2. Moreover, survival of those cases of refractory anemia with excess blasts-2 with, obviously, less than 50% bone marrow erythroblasts that, as in erythroleukemia, had equal or greater than 20% bone marrow blasts from nonerythoid cells was almost identical to de novo erythroleukemia. This finding underlines the arbitrariness of the established erythroblast cutoff to consider the diagnosis of erythroleukemia and the absence of prognostic impact per se of the erythroid predominance.
The upcoming revision of the WHO classification will consider erythroleukemias with equal or greater than 5% to less than 10% bone marrow blasts from total nucleated cells as myelodysplastic syndromes with excess blasts-1 and those with equal or greater than 10% to less than 20% bone marrow blasts from total nucleated cells as myelodysplastic syndromes with excess blasts-2.14, 15 In our study, we observed that de novo erythroleukemia patients with less than 10% bone marrow blasts from total nucleated cells (future myelodysplastic syndrome with excess blasts-1) presented a survival similar to patients with equal or greater than 10% to less than 20% bone marrow blasts from total nucleated cells (future myelodysplastic syndrome with excess blasts-2). Moreover, erythroid-predominant refractory anemia with excess blasts-1 showed a significant inferior overall survival than refractory anemia with excess blasts-1 with less than 50% bone marrow erythroblasts. This could be explained, at least in part, by the differences in the median percentage of bone marrow blasts calculated from nonerythroid cells. Although the median percentage of bone marrow blasts from total nucleated cells was higher in refractory anemia with excess blasts-1 with less than 50% bone marrow erythroblasts than in erythroid-predominant refractory anemia with excess blasts-1, the latter showed a significant higher median percentage of bone marrow blasts from nonerythroid cells. This finding highlights the importance of considering the percentage of bone marrow blasts from nonerythroid cells, especially in the subset of erythroid-rich myelodysplastic syndrome patients.11 For all the above, the new proposal of including erythroleukemia patients with equal or greater than 5% to less than 10% bone marrow blasts from total nucleated cells in the myelodysplastic syndrome with excess blasts-1 category could underestimate the prognostic prediction of this group of low-blast count erythroleukemias. The elimination of the nonerythroid blast cell count rule implies the disappearance of erythroleukemia as an entity. In our opinion, considering de novo erythroleukemia as a ‘high-risk’ myelodysplastic syndrome subgroup could be a proper approach to avoid the underestimation of those cases with a higher percentage of bone marrow erythroblasts and lower bone marrow blast count from total nucleated cells.
In summary, our data suggest that erythroleukemia is in the spectrum of myelodysplastic syndromes with excess blasts and support its inclusion into future myelodysplastic syndrome classifications rather than in acute myeloid leukemia ones. Classifying these poor outcome patients, usually elderly and unfit to receive common acute myeloid leukemia-type treatments, into myelodysplastic syndrome categories could permit them to benefit from a myelodysplastic syndrome-oriented prognostic assessment and could serve for their inclusion in future myelodysplastic syndrome-specific clinical trials.
References
Brunning RD, Bennett JM, Flandrin G et al. Myelodysplastic syndromes: introduction. In: Jaffe ES, Harris NL, Stein H (eds). World Health Organization Classification of Tumours of Hematopoietic and Lymphoid Tissues. IARC Press, World Health Organization: Lyon, France, 2001, pp 1–351.
Brunning RD, Orazi A, Germing U et al. Myelodysplastic syndromes. In: Swerdlow S, Campos E, Lee Harris N (eds). WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC press, World Health Organization: Lyon, France, 2008, pp 87–107.
Lessard M, Struski S, Leymarie V et al. Cytogenetic study of 75 erythroleukemias. Cancer Genet Cytogenet 2005:163:113–122.
Park S, Picard F, Guesnu M et al. Erythroleukemia and RAEB-t: a same disease? Leukemia 2004:18:888–890.
Cuneo A, van Orshoven A, Michaux JL et al. Morphologic, immunologic and cytogenetic studies in erythroleukaemia: evidence for multilineage involvement and identification of two distinct cytogenetic–clinicopathological types. Br J Haematol 1990:75:346–354.
Bacher U, Haferlach C, Alpermann T et al. Comparison of genetic and clinical aspects in patients with acute myeloid leukemia and myelodysplastic syndromes all with more than 50% of bone marrow erythropoietic cells. Haematologica 2011:96:1284–1292.
Grossmann V, Bacher U, Haferlach C et al. Acute erythroid leukemia (AEL) can be separated into distinct prognostic subsets based on cytogenetic and molecular genetic characteristics. Leukemia 2013:27:1940–1943.
Hasserjian RP, Zuo Z, Garcia C et al. Acute erythroid leukemia: a reassessment using criteria refined in the 2008 WHO classification. Blood 2010:115:1985–1592.
Santos FP, Faderl S, Garcia-Manero G et al. Adult acute erythroleukemia: an analysis of 91 patients treated at a single institution. Leukemia 2009:23:2275–2280.
Zuo Z, Medeiros LJ, Chen Z et al. Acute myeloid leukemia (AML) with erythroid predominance exhibits clinical and molecular characteristics that differ from other types of AML. PLoS One 2012:7:e41485.
Wang SA, Tang G, Fadare O et al. Erythroid-predominant myelodysplastic syndromes: enumeration of blasts from nonerythroid rather than total marrow cells provides superior risk stratification. Mod Pathol 2008:21:1394–1402.
Wong E, Juneja S . Acute myeloid leukemia and myelodysplastic syndromes with 50% or greater erythroblasts: a diagnostic conumdrun. Pathology 2015:47:289–293.
Mazzella FM, Smith D, Horn P et al. Prognostic significance of pronormoblasts in erythrocyte predominant myelodysplastic patients. Am J Haematol 2006:81:484–491.
Arber DA, Hasserjian RP . Reclassifying myelodysplastic syndromes: what's where in the new WHO and why. Hematol Am Soc Hematol Educ Program 2015:2015:294–298.
Arber DA, Orazi A, Hasserjian R et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016:127:2391–2405.
Bennett JM, Begg CB . Easter cooperative Oncology Group study off the cytochemistry of adult acute leukemia by correlations of subtypes with response and survival. Cancer Res 1981:41:4833–4837.
Bennett JM, Catovsky D, Daniel MT et al. Proposals for the classification of the acute leukemias. Br J Haematol 1976:33:451–458.
Bennett JM, Catovsky D, Daniel MT et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol 1982:51:189–199.
Bennett JM, Catovsky D, Daniel MT et al. Proposed revised criteria for the classification of acute leukemia. A report of the French-American-British Cooperative Group. Ann Intern Med 1985:103:620–625.
Grupo Español de Síndromes Mielodisplásicos (GESMD) and Sociedad Española de Hematología y Hemoterapia (SEHH). Guías españolas de diagnóstico y tratamiento de los síndromes mielodisplásicos y la leucemia mielomonocítica crónica. Haematol Esp 2012:97:11–14.
Shaffer L, Slovak M, Campbell L . ISCN2009: An International System for Human Cytogenetic Nomenclature. Karger AG: Basel, Switzerland, 2009.
Greenberg P, Cox C, LeBeau MM et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997:89:2079–2088.
Greenberg PL, Tuechler H, Schanz J et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012:120:2454–2465.
Acknowledgements
We thank Luis Benlloch and Ana Vicente by the maintenance of the Grupo Español de Síndromes Mielodisplásicos database. Institutions and investigators from the Grupo Español de Síndromes Mielodisplásicos that participated in the present study are listed in the Supplementary Information. This work has been partially supported by the following grants: RD12/0036/0044 from Red Temática de Investigación Cooperativa en Cáncer (RTICC, FEDER); and 2014/SGR585 from Agència de Gestió d’Ajuts Universitaris i de Recerca of Generalitat de Catalunya.
Author information
Authors and Affiliations
Consortia
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Presented in part in the 13th International Symposium on Myelodysplastic Syndromes (Washington, DC, USA, 29 April 2015 to 2 May 2015) and the 57th American Society of Hematology (ASH) annual meeting (Orlando, FL, USA, 5–8 December).
Supplementary Information accompanies the paper on Modern Pathology website
Supplementary information
Rights and permissions
About this article
Cite this article
Calvo, X., Arenillas, L., Luño, E. et al. Erythroleukemia shares biological features and outcome with myelodysplastic syndromes with excess blasts: a rationale for its inclusion into future classifications of myelodysplastic syndromes. Mod Pathol 29, 1541–1551 (2016). https://doi.org/10.1038/modpathol.2016.146
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2016.146