Abstract
Classical Hodgkin lymphoma and ALK− anaplastic large cell lymphoma share many features like strong CD30 expression and usually loss of B- and T-cell markers. However, their clinical course is dramatically different with curability rates of >90% for classical Hodgkin lymphoma and an unfavorable prognosis for anaplastic large cell lymphoma. Classical Hodgkin lymphoma and ALK− anaplastic large cell lymphoma can usually be distinguished by PAX5 expression in the Hodgkin and Reed-Sternberg cells of classical Hodgkin lymphoma and expression of cytotoxic molecules in tumor cells of anaplastic large cell lymphoma. However, in some cases the differential diagnosis is difficult owing to absence of established markers. To be able to better classify these cases, we reevaluated gene expression data of microdissected tumor cells of both lymphomas for differentially expressed genes. A classifier was established, comprising four genes strongly expressed in Hodgkin and Reed-Sternberg cells of classical Hodgkin lymphoma (MDC/CCL22, CD83, STAT3, and TUBB2B). Applying this classifier to a test cohort, Hodgkin lymphoma was successfully distinguished from ALK− anaplastic large cell lymphoma with an accuracy of 97% (43/44). MDC/CCL22, CD83, and STAT3 have also been found to be expressed in antigen-presenting cells. Therefore, based on our established classifier, Hodgkin and Reed-Sternberg cells differ from tumor cells of anaplastic large cell lymphoma, which can successfully be applied for practical purposes in histopathologic diagnostics.
Similar content being viewed by others
Main
Classical Hodgkin lymphoma and anaplastic large cell lymphoma are both characterized by the presence of CD30-positive tumor cells.1, 2 Furthermore, anaplastic large cell lymphoma is divided into ALK+ cases, presenting with a translocation affecting the ALK locus,3, 4, 5 and ALK− cases, in which translocations involving DUSP22 have been described in a fraction of cases.6 Whereas classical Hodgkin lymphoma often presents in young adults,2 anaplastic large cell lymphoma arises at various ages. ALK+ anaplastic large cell lymphoma is more frequently found in children and adolescents, whereas ALK− anaplastic large cell lymphoma is often observed in older patients.7, 8 Classical Hodgkin lymphoma can be cured in about 90% of patients with currently applied treatment approaches.9 However, ALK− anaplastic large cell lymphoma often shows poor outcome,10 particularly in the elderly. Therefore, a correct diagnostic classification of both diseases is mandatory for an appropriate therapeutic strategy.
Although classical Hodgkin lymphoma is a B-cell neoplasm, the tumor cells, Hodgkin, and Reed-Sternberg cells—have largely lost their B-cell phenotype.11, 12 Anaplastic large cell lymphoma is a T-cell neoplasm, but the tumor cells often present with a null phenotype and can be negative for all T-cell markers.13, 14 In ALK+ anaplastic large cell lymphoma immunohistochemical detection of ALK expression is a decisive marker for the differential diagnosis, but this marker cannot be applied for ALK− anaplastic large cell lymphoma. In some cases of anaplastic large cell lymphoma cytotoxic molecules like TIA1, perforin, and granzyme B are expressed.15 However, there are rare Hodgkin lymphoma cases in which expression of these cytotoxic molecules can also be found in Hodgkin and Reed-Sternberg cells.15, 16 In the past years, the B-cell transcription factor PAX5 has been discovered to be still expressed at low level in Hodgkin and Reed-Sternberg cells of most classical Hodgkin lymphoma cases.11, 17, 18 Classical Hodgkin lymphoma can therefore in most cases reliably be distinguished from ALK− anaplastic large cell lymphoma. However, some PAX5-negative classical Hodgkin lymphoma cases18 are difficult to be distinguished from ALK− anaplastic large cell lymphoma. Ancillary molecular studies showing rearrangements of B- or T-cell receptors can be helpful in these cases. However, owing to a low tumor cell content, mostly observed in Hodgkin lymphoma and sometimes also found in anaplastic large cell lymphoma, they do not always give indicative results.
Therefore, the aim of the present study was to identify a set of characteristic markers, which can successfully be applied in the differential diagnosis of classical Hodgkin lymphoma and ALK− anaplastic large cell lymphoma.
Materials and methods
Microarray Data Analysis and Selection of Target Genes
Gene expression data from the studies by Brune et al19 and Eckerle et al20 were reevaluated. A supervised comparison between ALK− anaplastic large cell lymphoma cases and all Hodgkin lymphoma cases from both studies was performed. After the global filtering, a two-sample t-test (assuming equal variance in both groups) was applied to identify genes that were differentially expressed between the two groups. To account for multiple testing, the false discovery rate was used, as described by Benjamini and Hochberg.21 For visualization, a heat map of all significantly upregulated or downregulated transcripts was created (P<0.05, false discovery rate <0.1). Genes to be immunohistochemically validated on the protein level were chosen with regard to the strongest upregulation, the most homogenous expression patterns in the lymphoma samples, and the commercial availability of antibodies.
Tissue Samples and Immunohistochemical Stainings
Thirty classical Hodgkin lymphoma cases (10 nodular sclerosing subtype, 10 mixed cellularity, and 10 Hodgkin lymphoma cases with expression of granzyme B—9 mixed cellularity and 1 lymphocyte depleted), 20 nodal ALK− anaplastic large cell lymphoma as well as 11 primary cutaneous ALK− anaplastic large cell lymphoma, diagnosed according to the World Health Organization 2008 classification,2 were selected from the archives of the Dr Senckenberg Department of Pathology in Frankfurt. We specifically selected granzyme B expressing Hodgkin lymphoma cases, because in classical Hodgkin lymphoma with PAX5,+ Hodgkin and Reed-Sternberg cells sometimes show coexpression of cytotoxic molecules, in particular granzyme B, thus sharing even more features with anaplastic large cell lymphoma. A validation cohort of 30 additional classical Hodgkin lymphoma cases, 13 ALK− and 11 ALK+ anaplastic large cell lymphoma cases was selected from the archives of the Department of Experimental, Diagnostic and Specialty Medicine, Haematopathology Section, S. Orsola-Malpighi Hospital, University of Bologna, Italy and stained on tissue microarray format. Stainings were considered positive if at least 50% of the tumor cells were positive. The local ethics committees of the Universities in Frankfurt and Bologna approved the study. Detailed information on the immunohistochemical profiles of the cases are found in Table 1. Whole tissue sections were stained for CD83, MDC/CCL2, TUBB2B, STAT3, and CAPN2 using the Peroxidase-FLEX EnVision Kit (Dako) as described previously.22, 23 The antibodies used, dilutions, and providers are listed in Table 2. Antigen unmasking was performed for 3 min in a pressure cooker or microwave in Tris-EDTA at pH 8.0 or citrate buffer at pH 6.0. All cases had previously been stained for CD20, CD30, CD3, PAX5, TIA1, granzyme B, and LMP1. Hierarchical cluster analysis was conducted on the four immunohistochemical stainings included in the classifier using R and Bioconductor.
Results
Gene Expression Analysis
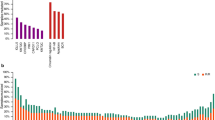
In a reevaluation of gene expression analyses performed by Brune et al19 and Eckerle et al20 a supervised comparison of the gene expression profiles of microdissected tumor cells of classical Hodgkin lymphoma and ALK− anaplastic large cell lymphoma was performed. Eighteen transcripts were found to be overexpressed in Hodgkin and Reed-Sternberg cells of classical Hodgkin lymphoma compared with the tumor cells of ALK− anaplastic large cell lymphoma and 15 transcripts were overexpressed in the tumor cells of ALK− anaplastic large cell lymphoma (fold change<−1.8 or >1.8, P-value<0.05, false discovery rate<0.1, Table 3, Figure 1). Based on significant differential expression, macrophage-derived chemokine/chemokine ligand 22 (MDC/CCL22) (39.8-fold upregulated in Hodgkin and Reed-Sternberg cells), tubulin beta 2B (TUBB2B) (9.9-fold upregulated in Hodgkin and Reed-Sternberg cells), CD83 (8.4-fold upregulated in Hodgkin and Reed-Sternberg cells), signal transducer and activator of transcription 3 (STAT3) (4.2-fold upregulated in Hodgkin and Reed-Sternberg cells), and calpain 2 (CAPN2) (5.0-fold upregulated in the tumor cells of ALK− anaplastic large cell lymphoma) were selected for immunohistochemical studies (Figure 1).
Heat map of the genes differentially expressed in the microdissected tumor cells of classical Hodgkin lymphoma and ALK− anaplastic large cell lymphoma from the studies by Brune et al19 and Eckerle et al.20 NA, not annotated; red color: high expression; black color: intermediate expression; green color: low expression. Genes selected for immunohistochemical studies are highlighted by arrows.
Immunohistochemical Investigation of Differentially Expressed Genes on Protein Level
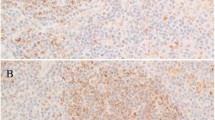
In majority of the 30 classical Hodgkin lymphoma cases of the first series, stained on whole tissue sections, Hodgkin and Reed-Sternberg cells were positive for TUBB2B (80%) as well as STAT3, CD83, and MDC/CCL22 (83% each, Table 1 and Figure 2). MDC/CCL22 and CD83 were strongly expressed in the cytoplasm and Golgi field of Hodgkin and Reed-Sternberg cells, whereas TUBB2B showed a homogeneous expression in the cytoplasm. An enhanced STAT3 expression was observed in the nucleus and cytoplasm of Hodgkin and Reed-Sternberg cells. A weak expression of STAT3 was also observed in histiocytes and the tumor cells of some anaplastic large cell lymphoma cases (Figures 2e and f). Only 10% of the 31 anaplastic large cell lymphoma in the first series presented with a strong nuclear and cytoplasmic STAT3 expression and cytoplasmic TUBB2B expression. Like the Hodgkin and Reed-Sternberg cells, a subgroup of macrophages/dendritic cells stained positive for CD83 and MDC/CCL22 in some cases, whereas anaplastic large cell lymphoma tumor cells were consistently negative in this first series. Interestingly, among 10 classical Hodgkin lymphoma cases with expression of granzyme B, Hodgkin and Reed-Sternberg cells were less frequently positive for the Hodgkin classifier markers, when compared with the typical classical Hodgkin lymphoma cases being negative for cytotoxic molecules. In these cases, MDC/CCL22 was positive in only 5/10 cases (50%), CD83 was positive in 7/10 cases (70%), TUBB2B and STAT3 were each positive in 8/10 cases (80%).
Immunhistochemical stainings differentiating classical Hodgkin lymphoma and anaplastic large cell lymphoma. (a and b) CD83; (c and d) MDC/CCL22; (e and f) STAT3; (g and h) TUBB2B; (i and j) CAPN2. (a, c, e and g) Hodgkin and Reed-Sternberg cells of classical Hodgkin lymphoma show expression of the respective immunohistochemical markers. (b, d, f and h) Tumor cells of ALK-negative anaplastic large cell lymphoma are negative for the respective markers. (i) Hodgkin and Reed-Sternberg cells are negative for CAPN2, whereas a subset of lymphocytes is positive. Inset: classical Hodgkin lymphoma case with CAPN2-positive Hodgkin and Reed-Sternberg cells. (j) Tumor cells of ALK− anaplastic large cell lymphoma show expression of CAPN2.
CAPN2 was expressed in the tumor cells of both classical Hodgkin lymphoma and ALK− anaplastic large cell lymphoma cases (23 and 45%, respectively) and was therefore not further considered.
In the training set, stained on tissue microarray, Hodgkin and Reed-Sternberg cells were also positive in majority of the 30 classical Hodgkin lymphoma cases for TUBB2B and MDC/CCL22 (77% each), STAT3 (93%), and CD83 (90%). In contrast, tumor cells of the 13 ALK− anaplastic large cell lymphoma cases were only infrequently positive for TUBB2B (8%), STAT3 (15%), and CD83 (31%). They were always negative for MDC/CCL22.
Establishment of a Classifier to Efficiently Distinguish Classical Hodgkin Lymphoma and ALK− Anaplastic Large Cell Lymphoma
A scoring system (0–4) was created reflecting the number of positive stainings (MDC/CCL22, CD83, STAT3, and TUBB2B) per case (0,no positive stainings; 4,all four stainings positive). The first case series, stained on whole tissue sections, was evaluated applying this scoring system. A threshold of ≥2-positive stainings proved to be optimal for the classification as Hodgkin lymphoma, whereas cases with a score 0–1 were regarded as anaplastic large cell lymphoma. Applying this scoring system to the second series of cases stained on tissue microarray, all 30 classical Hodgkin lymphoma cases were correctly classified and only one out of 13 ALK− anaplastic large cell lymphoma cases was misleadingly classified as Hodgkin lymphoma (42/43, 97%, Table 4). Applying the classifier to all cases, two out of 60 Hodgkin lymphoma cases and two out of 34 nodal ALK− anaplastic large cell lymphoma were misclassified, yielding an accuracy of 96% (90/94). The misclassified Hodgkin lymphoma cases were two cases with granzyme B expression. In an unsupervised hierarchical clustering by the classifier, two large branches were observed (Figure 3).
Unsupervised hierarchical clustering by the classifier of four immunohistochemical stainings for MDC/CCL22, CD83, STAT3, and TUBB2B. Red color: tumor cells positive, green color: tumor cells negative. Color code: yellow: classical Hodgkin lymphoma, green classical Hodgkin lymphoma with granzyme B expression, red: ALK− anaplastic large cell lymphoma, orange: cutaneous anaplastic large cell lymphoma, blue: ALK+ anaplastic large cell lymphoma.
Discussion
Several attempts have been made to establish classifiers differentiating between ALK− anaplastic large cell lymphoma and peripheral T-cell lymphoma not otherwise specified,24, 25, 26 but to our knowledge this has not been achieved for differential diagnosis of classical Hodgkin lymphoma and ALK− anaplastic large cell lymphoma. The aim of the present study was to find new immunohistochemical markers which can be applied in the differential diagnosis of ALK− anaplastic large cell lymphoma and classical Hodgkin lymphoma in daily routine practice. Our classifier was established by the reevaluation of gene expression studies of microdissected tumor cells and shows a high specificity and sensitivity.
Owing to overexpression of several genes in the Hodgkin and Reed-Sternberg cells of classical Hodgkin lymphoma we were able to distinguish between ALK− anaplastic large cell lymphoma and classical Hodgkin lymphoma. The one gene overexpressed in ALK− anaplastic large cell lymphoma did not prove to be diagnostically powerful. One of the genes upregulated in classical Hodgkin lymphoma was TUBB2B, a member of the beta-tubulin family, involved in the formation of microtubuli and therefore has a role in several important cellular processes, including mitosis. TUBB2B has so far not been described to be upregulated in Hodgkin and Reed-Sternberg cells, whereas another member of the beta-tubulin family, TUBB3, was observed to be expressed in both the tumor cells of classical Hodgkin lymphoma and anaplastic large cell lymphoma.27 Recently, refusion of Hodgkin and Reed-Sternberg cells with a persisting microtubule bond between daughter cells could be demonstrated to be an important feature of giant cell formation.28 However, the reason of a selective tubulin overexpression in the Hodgkin and Reed-Sternberg cells as demonstrated in this study so far remains obscure.
The remaining significantly overexpressed genes are related to the interactions between Hodgkin and Reed-Sternberg cells and their microenvironment. CD83 is usually expressed in mature dendritic cells29 and triggers CD4+ T-cell maturation, but is also expressed by activated B cells and involved in the modulation of B-cell receptor signaling.30 CD83 has previously been described to be upregulated in Hodgkin and Reed-Sternberg cells of Hodgkin lymphoma.31 Transgenic overexpression of CD83 in B cells resulted in an upregulation of the major histocompatibility complex II (MHC II) and interleukin 10.30 However, MHC II is shown to be downregulated in a subset of classical Hodgkin lymphoma cases,32 partly owing to translocations involving the MHC II transactivator (CIITA).33 Decreased MHC class II expression has been linked to reduced tumor cell immunogenicity. A strong expression of STAT3 was observed in Hodgkin and Reed-Sternberg cells in the present and previous studies.34, 35, 36 STAT3 is mainly expressed in macrophages and dendritic cells and can exhibit—depending on the stimulating cytokine—both pro- and antiinflammatory properties.37 However, STAT3 is also found to be expressed in ALK+ anaplastic large cell lymphoma.38 In contrast, pediatric cases of ALK− anaplastic large cell lymphoma were negative for phosphorylated STAT3.39 Our results fit well with the observations made by Piva et al,26 when considering the number of ALK− anaplastic large cell lymphoma strongly expressing phosphorylated STAT3 in most if not all neoplastic cells. This can also be observed on RNA level in the gene expression arrays (Figure 1). However, it is important to point out, that in the present study only the amount of STAT3 protein was assessed, whereas phosphorylation and activation status were not evaluated. MDC/CCL22 is usually also expressed by antigen-presenting cells and is upregulated by TH2-type cytokines.40 It was shown to be expressed in Hodgkin and Reed-Sternberg cells of classical Hodgkin lymphoma41 and age-related Epstein–Barr virus-associated B-cell lymphoproliferative disorders.42 MDC/CCL22 and TARC/CCL17 share the receptor CCR4, by which TH2 and regulatory T cells can be attracted by Hodgkin and Reed-Sternberg cells.43, 44 However, it could recently be shown that the majority of T cells in classical Hodgkin lymphoma microenvironment represents TH1 cells.45
In conclusion, three of four genes overexpressed in the Hodgkin and Reed-Sternberg cells in comparison with the tumor cells of ALK− anaplastic large cell lymphoma are also expressed in antigen-presenting cells and are related to the interactions between Hodgkin and Reed-Sternberg cells and surrounding T cells. Moreover, Hodgkin and Reed-Sternberg cells are known to express several additional markers usually expressed by antigen-presenting cells.46, 47, 48Although Hodgkin and Reed-Sternberg cells have usually lost their B-cell phenotype, they have maintained the features for an interaction with T cells, which is not observed in the tumor cells of ALK− anaplastic large cell lymphoma. Interestingly, Hodgkin lymphoma cases with granzyme B expression were less frequently positive for MDC/CCL22 and CD83 than conventional classical Hodgkin lymphoma cases. These cases may therefore differently interact with their microenvironment and may be closely related to anaplastic large cell lymphoma.
In summary, the genes identified in the present study reflect a different pathogenesis of classical Hodgkin lymphoma and ALK− anaplastic large cell lymphoma. By closely interacting with surrounding T cells, Hodgkin and Reed-Sternberg cells have maintained parts of their B-cell identity and show a fundamentally different interaction with their microenvironment than tumor cells in ALK− anaplastic large cell lymphoma. These features can be practically applied in the diagnostic distinction between classical Hodgkin lymphoma and ALK− anaplastic large cell lymphoma. However, owing to the limited case number investigated in the present study, our newly established classifier should be validated in a large patient series with clinical data.
References
Stein H, Mason DY, Gerdes J et al. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood 1985;66:848–858.
Swerdlow SH International Agency for Research on Cancer, World Health Organization. WHO classification of tumours of haematopoietic and lymphoid tissues 4th edn International Agency for Research on Cancer: Lyon, France, 2008, pp 317–334.
Mason DY, Bastard C, Rimokh R et al. CD30-positive large cell lymphomas ('Ki-1 lymphoma') are associated with a chromosomal translocation involving 5q35. Br J Haematol 1990;74:161–168.
Lamant L, Meggetto F, al Saati T et al. High incidence of the t(2;5)(p23;q35) translocation in anaplastic large cell lymphoma and its lack of detection in Hodgkin's disease. Comparison of cytogenetic analysis, reverse transcriptase-polymerase chain reaction, and P-80 immunostaining. Blood 1996;87:284–291.
Morris SW, Kirstein MN, Valentine MB et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science 1994;263:1281–1284.
Feldman AL, Dogan A, Smith DI et al. Discovery of recurrent t(6;7)(p25.3;q32.3) translocations in ALK-negative anaplastic large cell lymphomas by massively parallel genomic sequencing. Blood 2011;117:915–919.
Savage KJ, Harris NL, Vose JM et al. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood 2008;111:5496–5504.
Pileri SA, Agostinelli C, Bacci F et al. Pathobiology of ALK-negative anaplastic large cell lymphoma. Pediatr Rep 2011;3 (Suppl 2):e5.
Eichenauer DA, Engert A . Advances in the treatment of Hodgkin lymphoma. Int J Hematol 2012;96:535–543.
Vose J, Armitage J, Weisenburger D . International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol 2008;26:4124–4130.
Schwering I, Bräuninger A, Klein U et al. Loss of the B-lineage-specific gene expression program in Hodgkin and Reed-Sternberg cells of Hodgkin lymphoma. Blood 2003;101:1505–1512.
Küppers R, Engert A, Hansmann ML . Hodgkin lymphoma. J Clin Invest 2012;122:3439–3447.
Bonzheim I, Geissinger E, Roth S et al. Anaplastic large cell lymphomas lack the expression of T-cell receptor molecules or molecules of proximal T-cell receptor signaling. Blood 2004;104:3358–3360.
Geissinger E, Sadler P, Roth S et al. Disturbed expression of the T-cell receptor/CD3 complex and associated signaling molecules in CD30+ T-cell lymphoproliferations. Haematologica 2010;95:1697–1704.
Foss HD, Anagnostopoulos I, Araujo I et al. Anaplastic large-cell lymphomas of T-cell and null-cell phenotype express cytotoxic molecules. Blood 1996;88:4005–4011.
Asano N, Kinoshita T, Tamaru J et al. Cytotoxic molecule-positive classical Hodgkin's lymphoma: a clinicopathological comparison with cytotoxic molecule-positive peripheral T-cell lymphoma of not otherwise specified type. Haematologica 2011;96:1636–1643.
Foss HD, Reusch R, Demel G et al. Frequent expression of the B-cell-specific activator protein in Reed-Sternberg cells of classical Hodgkin's disease provides further evidence for its B-cell origin. Blood 1999;94:3108–3113.
Agostinelli C, Sabattini E, Gjorret JO et al. Characterization of a New Monoclonal Antibody Against PAX5/BASP in 1525 Paraffin-embedded Human and Animal Tissue Samples. Appl Immunohistochem Mol Morphol 2010;18:561–572.
Brune V, Tiacci E, Pfeil I et al. Origin and pathogenesis of nodular lymphocyte-predominant Hodgkin lymphoma as revealed by global gene expression analysis. J Exp Med 2008;205:2251–2268.
Eckerle S, Brune V, Doring C et al. Gene expression profiling of isolated tumour cells from anaplastic large cell lymphomas: insights into its cellular origin, pathogenesis and relation to Hodgkin lymphoma. Leukemia 2009;23:2129–2138.
Benjamini Y, Hochberg Y . Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc 1995;57:289–300.
Hartmann S, Tousseyn T, Döring C et al. Macrophages in T cell/histiocyte rich large B cell lymphoma strongly express metal-binding proteins and show a bi-activated phenotype. Int J Cancer 2013;133:2609–2618.
Hartmann S, Agostinelli C, Klapper W et al. Revising the historical collection of epithelioid cell-rich lymphomas of the Kiel Lymph Node Registry: what is Lennert's lymphoma nowadays? Histopathology 2011;59:1173–1182.
Agnelli L, Mereu E, Pellegrino E et al. Identification of a 3-gene model as a powerful diagnostic tool for the recognition of ALK-negative anaplastic large-cell lymphoma. Blood 2012;120:1274–1281.
Piccaluga PP, Fuligni F, De Leo A et al. Molecular profiling improves classification and prognostication of nodal peripheral T-cell lymphomas: results of a phase III diagnostic accuracy study. J Clin Oncol 2013;31:3019–3025.
Piva R, Agnelli L, Pellegrino E et al. Gene expression profiling uncovers molecular classifiers for the recognition of anaplastic large-cell lymphoma within peripheral T-cell neoplasms. J Clin Oncol 2010;28:1583–1590.
Yoon SO, Kim WY, Go H et al. Class III beta-tubulin shows unique expression patterns in a variety of neoplastic and non-neoplastic lymphoproliferative disorders. Am J Surg Pathol 2010;34:645–655.
Rengstl B, Newrzela S, Heinrich T et al. Re-fusion of small mononucleated Hodgkin cells leads to giant multinucleated Reed-Sternberg cells in Hodgkin lymphoma. Proc Natl Acad Sci USA 2013;110:20729–20734.
Zhou LJ, Tedder TF . Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol 1995;154:3821–3835.
Breloer M, Fleischer B . CD83 regulates lymphocyte maturation, activation and homeostasis. Trends Immunol. 2008;29:186–194.
Sorg UR, Morse TM, Patton WN et al. Hodgkin's cells express CD83, a dendritic cell lineage associated antigen. Pathology 1997;29:294–299.
Diepstra A, van Imhoff GW, Karim-Kos HE et al. HLA class II expression by Hodgkin Reed-Sternberg cells is an independent prognostic factor in classical Hodgkin's lymphoma. J Clin Oncol 2007;25:3101–3108.
Steidl C, Shah SP, Woolcock BW et al. MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nature 2011;471:377–381.
Kube D, Holtick U, Vockerodt M et al. STAT3 is constitutively activated in Hodgkin cell lines. Blood 2001;98:762–770.
Holtick U, Vockerodt M, Pinkert D et al. STAT3 is essential for Hodgkin lymphoma cell proliferation and is a target of tyrphostin AG17 which confers sensitization for apoptosis. Leukemia 2005;19:936–944.
Lamprecht B, Kreher S, Anagnostopoulos I et al. Aberrant expression of the Th2 cytokine IL-21 in Hodgkin lymphoma cells regulates STAT3 signaling and attracts Treg cells via regulation of MIP-3alpha. Blood 2008;112:3339–3347.
Braun DA, Fribourg M, Sealfon SC . Cytokine response is determined by duration of receptor and signal transducers and activators of transcription 3 (STAT3) activation. J Biol Chem 2013;288:2986–2993.
Anastasov N, Bonzheim I, Rudelius M et al. C/EBPbeta expression in ALK-positive anaplastic large cell lymphomas is required for cell proliferation and is induced by the STAT3 signaling pathway. Haematologica 2010;95:760–767.
Nasr MR, Laver JH, Chang M et al. Expression of anaplastic lymphoma kinase, tyrosine-phosphorylated STAT3, and associated factors in pediatric anaplastic large cell lymphoma: a report from the children's oncology group. Am J Clin Pathol 2007;127:770–778.
Yamashita U, Kuroda E . Regulation of macrophage-derived chemokine (MDC, CCL22) production. Crit Rev Immunol 2002;22:105–114.
Hanamoto H, Nakayama T, Miyazato H et al. Expression of CCL28 by Reed-Sternberg cells defines a major subtype of classical Hodgkin's disease with frequent infiltration of eosinophils and/or plasma cells. Am J Pathol 2004;164:997–1006.
Takegawa S, Jin Z, Nakayama T et al. Expression of CCL17 and CCL22 by latent membrane protein 1-positive tumor cells in age-related Epstein-Barr virus-associated B-cell lymphoproliferative disorder. Cancer Sci 2008;99:296–302.
Marshall NA, Christie LE, Munro LR et al. Immunosuppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood 2004;103:1755–1762.
Ishida T, Ishii T, Inagaki A et al. Specific recruitment of CC chemokine receptor 4-positive regulatory T cells in Hodgkin lymphoma fosters immune privilege. Cancer Res 2006;66:5716–5722.
Greaves P, Clear A, Owen A et al. Defining characteristics of classical Hodgkin lymphoma microenvironment T-helper cells. Blood 2013;122:2856–2863.
Lamprecht B, Walter K, Kreher S et al. Derepression of an endogenous long terminal repeat activates the CSF1R proto-oncogene in human lymphoma. Nat Med 2010;16:571–579 571p following 579.
Steidl C, Diepstra A, Lee T et al. Gene expression profiling of microdissected Hodgkin Reed Sternberg cells correlates with treatment outcome in classical Hodgkin lymphoma. Blood 2012;120:3530–3540.
Hartmann S, Tousseyn T, Doring C et al. Macrophages in T cell/histiocyte rich large B cell lymphoma strongly express metal-binding proteins and show a bi-activated phenotype. Int J Cancer 2013;133:2609–2618.
Acknowledgements
We would like to thank Sabine Albrecht for excellent technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft (NE-1438/4-1 as part of the FOR-1961 collaborative research group on mature T-cell lymphomas ‘CONTROL-T’, and KU1315/7-1) and AIRC 5 × 1000 (Grant 10007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Döring, C., Hansmann, ML., Agostinelli, C. et al. A novel immunohistochemical classifier to distinguish Hodgkin lymphoma from ALK anaplastic large cell lymphoma. Mod Pathol 27, 1345–1354 (2014). https://doi.org/10.1038/modpathol.2014.44
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2014.44