Abstract
BMI-1 is a member of the Polycomb group of genes (PcGs) and is involved in embryonic gene regulation and maintenance of adult stem cells. It has been suggested that BMI-1 protein is important in cell cycle regulation, since both p16/INK4a and p14/ARF are downstream BMI-1 targets. BMI-1 has been implicated in the development and progression of several malignancies, but its role in melanocytic tumors of the skin is unknown. In the present study, using immunohistochemistry on 178 benign and malignant melanocytic lesions and two different antibodies, BMI-1 expression was reduced in melanomas compared with benign nevi. In established melanomas, loss of BMI-1 expression was associated with features of aggressive tumors, such as increased tumor cell proliferation, presence of necrosis and increased expression of both N-cadherin and β3-integrin, indicating a more invasive and mesenchymal phenotype. Low BMI-1 expression was associated with low p14 and CDK4 but not with p16 expression. Low levels of BMI-1 expression were also significantly associated with decreased patient survival.
Similar content being viewed by others
Main
The incidence of cutaneous melanoma is increasing,1 and there is a continuous search for improved prognostic markers and novel therapeutic approaches. BMI-1, a candidate stem cell marker, is part of the Polycomb group of genes (PcGs), which are important in embryonic gene regulation2 and tumor development.3 The PcG proteins are organized into two major complexes: PRC1 or maintenance of repression complex, which includes BMI-1, Mel-18, HPC, M33 and RING1 among others, and PRC2 or induction of repression complex, where one of the participants is EZH2.2, 4 BMI-1 is essential for blood-cell development and is highly expressed in hematopoietic stem cells,5 and it is also important for cell cycle regulation since both p16/INK4a and p14/ARF are downstream targets of BMI-1.2, 5, 6, 7 EZH2 is downstream of the RB–E2F pathway4 and is involved in cell cycle regulation through mechanisms other than BMI-1.
Expression of both BMI-1 and EZH2 has been associated with malignant tumors of the hematopoietic and lymphatic systems,8, 9, 10 and EZH2 expression was recently associated with tumor cell proliferation and patient prognosis in melanoma and other solid cancers.11, 12, 13 In contrast, the role of BMI-1 in melanocytic tumors remains unresolved. Experimental studies convincingly link BMI-1 overexpression to carcinogenesis, and it was recently reported that high expression of BMI-1 in nasopharyngeal epithelial cancers was associated with poor patient outcome.14 Glinsky et al15 described the prognostic impact of a 11-gene signature including BMI-1 in a panel of epithelial cancers. In contrast, in breast cancer, BMI-1 expression was associated with estrogen and progesterone receptor positivity, but also with the presence of axillary lymph node metastases,16, 17 indicating a diverse role of BMI-1. Basic studies have suggested a role for BMI-1 by immortalization of mammary epithelial cells through induction of telomerase activity and regulation of cancer cells with stem-cell like properties and tumorigenic capacity.18, 19 In pilot studies of lung and colorectal cancers and pediatric brain tumors, however, BMI-1 was expressed in the majority of tumors, although no impact on proliferation or patient survival was observed.20, 21, 22, 23 In other studies, BMI-1 was found to be upregulated in samples of brain tumors, but there was no association to histological type.24 On this background, the aim of our study was to investigate the role of BMI-1 expression in a panel of melanocytic skin tumors with focus on tumor cell proliferation and cell cycle regulation, other markers of aggressive melanoma, and patient outcome. This was of interest since we have previously reported that p16 is involved in melanoma progression,25, 26 and BMI-1 is a known p16 repressor.3, 6 Here, we demonstrate for the first time that loss of BMI-1 expression is associated with melanoma development, aggressive tumor subgroups and reduced patient survival, with a suggested influence of BMI-1 on cell cycle regulation and alterations of cell adhesion molecules in this tumor lineage.
Materials and methods
Patients
The patient material of this series is described in detail elsewhere.25 Briefly, 202 cases of vertical growth phase melanoma of the nodular type were included (median age 64.4 years, median thickness 3.6 mm). The presence of a vertical growth phase and the lack of a radial growth phase, that is, adjacent in situ or microinvasive component, were used as inclusion criteria. There was no history of familial occurrence. In addition, 58 paired metastases (local skin, regional lymph nodes, distant) were examined.
Clinico-pathological characteristics and survival data have previously been reported, and information on proliferation,25 cell cycle regulators,25, 26, 27 EZH2 expression12 and angiogenesis28 was included for comparison.
Complete information on patient survival and time and cause of death was available in all 202 cases. Last date of follow-up was December 31, 1999, and median follow-up time for survivors was 89 months (range 24–221). Recurrence-free survival was available in 167 of 202 patients. During the follow-up period, 72 patients (36%) died of malignant melanoma and 45 (22%) died of other causes. Of the 167 radically treated patients with data on recurrence-free survival, 74 (44%) had recurrent disease. In this particular study, cases with sufficient material left in the tissue microarray (TMA) blocks (n=127) were examined for the expression of BMI-1, using two different antibodies.
In addition to this series of nodular melanoma, where our purpose was to examine the expression pattern and prognostic impact of BMI-1, 31 cases of benign melanocytic nevi (median age 26.6 years) and 20 cases of invasive superficial spreading melanomas (SSMs; median age 49.0 years, median thickness 1.7 mm) were included to examine associations between BMI-1 and different stages of melanocytic tumor progression. The Norwegian Data Inspectorate and the Regional Committee for Ethics in Research (Health Region III) have approved this study. The study was performed in accordance with the Declaration of Helsinki.
Clinico-pathological Variables
The following variables were recorded: date of histological diagnosis, sex, age at diagnosis, anatomical site of the primary tumor and presence of metastases at diagnosis (local, regional, distant). The hematoxylin and eosin (H&E)-stained slides were previously re-examined, and the following histological features were included: tumor thickness according to Breslow,29 level of invasion according to Clark et al,30 microscopic tumor ulceration, vascular invasion28 and mitotic count (number of mitotic figures per mm2, recorded at the base of the tumors).
Tissue Microarray
The TMA technique has previously been described and validated in several studies.31, 32, 33 Three tissue cylinders with a diameter of 0.6 mm32, 33 from representative tumor areas on H&E-stained slides, generally at the suprabasal areas of the primary tumors, were punched and mounted into a recipient paraffin block using a custom-made precision instrument (Beecher Instruments, Silver Spring, MD, USA). Sections of the resulting TMA blocks (5 μm) were then made by standard technique.
Immunohistochemistry
The immunohistochemical staining was performed on thin TMA sections (5 μm) of paraffin-embedded archival tissue. The slides were dewaxed with xylene/ethanol before microwave antigen retrieval for 10 min at 750 W and 15 min at 350 W in TE 9 buffer (pH 9). The slides were incubated overnight at 4°C with a monoclonal BMI-1 antibody (clone F6; Upstate, Lake Placid, NY, USA) diluted 1:800. The staining procedure was performed using the EnVision labelled polymer method, with a commercial kit (Dako Cytomation, Copenhagen, Denmark), with 3-amino-9-ethylcarbazole peroxidase as substrate before brief counterstaining with Mayer's hematoxylin. Slides incubated with mouse IgG1 diluted 1:800 were used as negative controls. Samples of breast and prostate cancers with known BMI-1 positivity were added as positive controls. This antibody was examined by western blot.
The TMA sections were also stained with a well-described non-commercial monoclonal anti-BMI-1 antibody.8, 10, 21, 34, 35 After pretreatment and antigen retrieval as described above, the slides were incubated for 60 min at room temperature with the undiluted monoclonal antibody (6C9) before completion of the staining procedure using the Catalyzed Signal Amplification System (Dako Cytomation) in line with the instructions from the manufacturer.
The arrays were scored blindly and there was good correlation between the two investigated antibodies (Spearman's rho correlation coefficient 0.62, P<0.0001).
Evaluation of Staining
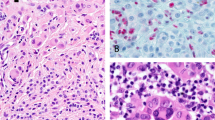
The immunohistochemical staining of BMI-1 showed a predominately nuclear staining pattern. The staining was recorded using a semiquantitative and subjective grading, considering both the intensity of staining and the proportion of tumor cells showing unequivocal positive reaction. A staining index (SI) was calculated as a product of staining intensity (0–3) and area of positive tumor cell nuclei (1, <10%; 2, 10–50%; 3, >50%).12 Evaluation of the cases was performed blinded for patient characteristics and outcome. In subsequent statistical analyses, the cutoff was based on median SI (<3 vs >3), after considering the frequency distribution curve and size of subgroups.
Western Blot
A 1:1 mixture of total HeLa cell lysate (the supernatant) and 2 × sample buffer (0.25 M Tris-HCl pH 6.8, 4% SDS, 20% glycerol, 0.05% bromophenol blue) and 5% β-mercaptoethanol were boiled for 5 min and the proteins were separated in a 10% acrylamide SDS-PAGE gel, using 15 μl/lane and transferred to a nitrocellulose membrane (Bio-Rad) at 100 mA for an hour in Tris-glycine buffer. The membranes were then blocked in 5% dry milk with 0.05% Tween 20 and incubated with the BMI-1 antibody (clone F6; Upstate). Labelled polymer-HRP anti-mouse (Envision, Dako Cytomation) was added in a 1:50 dilution. The bands were visualized by ECL detection. Our results indicate that the antibody is specific as it presented a distinct signal at the expected molecular weight of BMI-1 (MW≈40 kDa) (Figure 1).
Statistics
Analyses were performed using the SPSS statistical package, version 14.0 (SPSS Inc., Chicago, IL, USA). Associations between different categorical variables were assessed by Pearson's χ2 test. Continuous variables not following the normal distribution were compared between two or more groups using the Mann–Whitney U-test or Kruskal–Wallis H-tests. Wilcoxon signed rank test was used to compare related samples. Nonparametric correlations were tested with the Spearman's rank coefficient. Univariate analyses of time to death due to malignant melanoma or time to recurrence (recurrence-free survival) were performed using the product-limit procedure (Kaplan–Meier method), and differences between categories were estimated by the log-rank test, with date of histological diagnosis as the starting point. Patients who died of other causes were censored at the date of death. The influence of covariates on patient survival and recurrence-free survival was analyzed by the proportional hazards method and tested by the likelihood ratio (lratio) test.
Results
Melanocytic Nevi and Melanomas
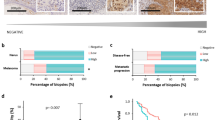
BMI-1 expression (F6 antibody) was significantly weaker in melanomas (superficial and nodular, n=146) when compared with benign melanocytic nevi (n=31) (P<0.0001). All SSMs (n=19) had a lower SI than benign nevi (P<0.0001) and also lower than nodular melanoma (P=0.014). Melanocytes in normal epidermis showed positive staining for BMI-1, with intensity comparable to what was seen in benign melanocytic nevi.
When analyzing the non-commercial antibody (6C9), loss of BMI-1 expression from nevi to melanomas was again demonstrated (P<0.0001), and SSMs showed weaker BMI-1 staining than did benign nevi (P<0.0001) and nodular melanomas (P=0.044).
Primary Nodular Melanoma
In the aggressive subgroup of nodular melanoma, proliferation (by mitotic count) was significantly higher (ratio 2.1) in tumors expressing low levels of BMI-1 (F6) (median of 8.2 mitoses/mm2) as compared with tumors showing strong BMI-1 staining (median 3.9 mitosis/mm2) (P=0.028). The nuclear staining pattern is shown in Figure 2. Strong expression of BMI-1 was also associated with strong staining of p14/ARF (P=0.001) and CDK4 (P=0.049), and weak/negative p53 expression (P=0.044). There was no significant association with p16 expression.
Loss of BMI-1 staining was associated with strong expression of cell adhesion molecules N-cadherin (P=0.004) and β3-integrin (P=0.013). Vascular activation in terms of microvessel density was found to be more pronounced in cases with strong BMI-1 expression (median 159.4 vessels/mm2) than in cases with low BMI-1 staining (median 118.8 vessels/mm2) (P=0.012), whereas tumors with evident necrosis expressed lower levels of BMI-1 (P=0.007). There was no significant association with tumor thickness, Clark's level of invasion, tumor ulceration or vascular invasion (Table 1).
When using the non-commercial BMI-1 antibody (6C9), similar results were obtained. In short, the proliferation rate was significantly higher among tumors expressing low levels of BMI-1, both assessed by mitotic counts (ratio 3.3, median 8.6 mitoses/mm2 compared to 2.6 mitoses/mm2 in tumors expressing high BMI-1 levels) (P=0.024) and Ki-67 (35 vs 25% positive tumor cells) (P=0.020). Loss of BMI-1 staining was associated with the presence of tumor necrosis (P=0.006) and with strong expression of β3-integrin (P=0.009), as well as with low levels of p14ARF (P=0.016) and CDK4 (P=0.004) expression (Table 1).
Primary and Metastatic Melanoma
When comparing BMI-1 expression in primary nodular melanomas with their corresponding metastases, 38 pairs had sufficient material and were available for this study. BMI-1 staining was found to be slightly stronger in metastases than in corresponding primary tumors (P=0.046 and P=0.023, analyzed with the commercial and non-commercial antibodies, respectively).
Survival Analyses
Low levels of BMI-1 expression were significantly associated with decreased patient survival in univariate analyses, confirmed in analyses with the two different antibodies described above. The 5-year survival rate was 51% in cases expressing low levels of BMI-1 compared to 83% in cases expressing high levels (P=0.006) (non-commercial antibody) (Figure 3), and the corresponding data for the commercial antibody were 56 and 75% (P=0.043) (Table 2).
In multivariate analyses, BMI-1 remained an independent prognostic factor (P=0.024) (non-commercial BMI-1 antibody) when included along with prognostic variables (Table 2) such as Breslow's tumor thickness, Clark's level of invasion, tumor ulceration, vascular invasion, proliferation (Ki-67) and p16 and CDK4 expression. The final multivariate model is shown in Table 3. BMI-1 expression showed a borderline significance in multivariate analyses when including the F6 antibody (P=0.11).
Discussion
The PcGs are major regulators of embryonic development as transcriptional repressors, and they are also important in the maintenance of adult stem cells.19, 36 Recently, more than 1000 genes bound by PcG proteins were identified by genome-wide mapping, among them key members of the Wnt, TGFβ, FGF, Notch and Hedgehog signalling pathways,36 thus illustrating the complexity by which PcGs regulate cell proliferation and differentiation. PcG proteins have also been promoted as targets for novel treatment strategies.5, 37 As members of the Polycomb repressor complexes, both EZH2 and BMI-1 are important in cell cycle regulation—EZH2 is located downstream and transcriptionally regulated by the Rb/E2F pathway, and BMI-1 is an upstream regulator of the CDKN2A locus.3
The importance of BMI-1 for tumor progression in the melanocytic lineage has not been previously studied. Here, we found that BMI-1 expression was significantly reduced in melanomas when compared with benign melanocytic nevi. This observation might support a tumor-suppressive role of BMI-1 in the melanocytic system, and suggests that BMI-1 loss may possibly contribute to the development of melanocytic tumors. BMI-1 has been proposed as a marker of cancer-initiating stem cells5 with a capacity for both renewal and differentiation,38 but it is not known whether loss of stem cell control might be involved in melanoma development. It should be mentioned that BMI-1 is also described as a marker of differentiation expressed in normal resting cells, as opposed to EZH2, which is mainly expressed in poorly differentiated cells of invasive carcinomas.39
In established melanomas, we found that loss of BMI-1 expression was significantly associated with increased tumor cell proliferation both by mitotic rate (2.1-fold increase) and Ki-67, as well as with the presence of tumor necrosis, both features of aggressive tumors. The mechanism for this observed loss of BMI-1 expression in a subgroup of nodular melanoma is not known. Strong expression of BMI-1 was associated with higher levels of p14, indicating that BMI-1 may act through a mechanism involving the p14–p53 pathway. Based on our findings, BMI-1 does not suppress p16 expression in these nodular melanomas, but rather induces or maintains p14 transcription and thereby contributes to the reduced growth capacity of tumor cells. The lack of association between BMI-1 expression and p16 status has also been indicated in breast cancer,17 lung cancer,40 Hodgkin's lymphoma8 and oral cancer.41 As mentioned, Bracken et al36 identified a large number of putative PcG target genes, supporting that BMI-1 may also exert its action in a p16-independent manner.
In a previous study, we reported that strong expression of EZH2 was associated with increased tumor cell proliferation and poor patient outcome in melanoma and other solid cancers,12 suggesting different roles of the Polycomb repressor complexes in these tumors. It is possible that target genes regulated by the PcGs EZH2 and BMI-1 play opposite roles in melanoma development and progress.
The progression of melanocytic tumors is associated with alterations in cell contact proteins, and experimental studies have indicated that a ‘switch’ from E-cadherin to N-cadherin expression is taking place during melanoma development and progress,42, 43 indicating the presence of epithelial to mesenchymal transition. We previously reported upregulation of P-cadherin expression in aggressive melanomas with poor prognosis,44 and we also demonstrated a strong expression of αv-integrin in the same subset of invasive melanomas.45 Others have suggested that expression of αvβ3 integrin promotes tumor matrix invasion by regulating MMP-2.46 In our study, loss of BMI-1 expression was associated with a strong staining of both N-cadherin and β3-integrin, indicating that BMI-1 could possibly act through alterations of cell adhesion molecules in this tumor cell lineage, and not solely by deregulation of the cell cycle. The explanation for this association is presently not known, but should be further studied, considering also the broad spectrum of PcG-targeted genes reported.36
In conclusion, we demonstrate for the first time that loss of BMI-1 expression is associated with increased proliferative capacity and reduced patient survival in established vertical growth phase melanomas. Our findings underscore the need for further studies to explore the role of BMI-1 in melanoma development and progress, and the possible relevance of this pathway for novel treatment strategies.
References
Garbe C, McLeod GR, Buettner PG . Time trends of cutaneous melanoma in Queensland, Australia and Central Europe. Cancer 2000;89:1269–1278.
Jacobs JJ, van Lohuizen M . Polycomb repression: from cellular memory to cellular proliferation and cancer. Biochim Biophys Acta 2002;1602:151–161.
Pasini D, Bracken AP, Helin K . Polycomb group proteins in cell cycle progression and cancer. Cell Cycle 2004;3:396–400.
Bracken AP, Pasini D, Capra M, et al. EZH2 is downstream of the pRB–E2F pathway, essential for proliferation and amplified in cancer. EMBO J 2003;22:5323–5335.
Raaphorst FM . Self-renewal of hematopoietic and leukemic stem cells: a central role for the Polycomb-group gene Bmi-1. Trends Immunol 2003;24:522–524.
Jacobs JJ, Kieboom K, Marino S, et al. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature 1999;397:164–168.
Molofsky AV, He S, Bydon M, et al. Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev 2005;19:1432–1437.
Dukers DF, van Galen JC, Giroth C, et al. Unique polycomb gene expression pattern in Hodgkin's lymphoma and Hodgkin's lymphoma-derived cell lines. Am J Pathol 2004;164:873–881.
Raaphorst FM, van Kemenade FJ, Blokzijl T, et al. Coexpression of BMI-1 and EZH2 polycomb group genes in Reed–Sternberg cells of Hodgkin's disease. Am J Pathol 2000;157:709–715.
van Kemenade FJ, Raaphorst FM, Blokzijl T, et al. Coexpression of BMI-1 and EZH2 polycomb-group proteins is associated with cycling cells and degree of malignancy in B-cell non-Hodgkin lymphoma. Blood 2001;97:3896–3901.
Steele JC, Torr EE, Noakes KL, et al. The polycomb group proteins, BMI-1 and EZH2, are tumour-associated antigens. Br J Cancer 2006;95:1202–1211.
Bachmann IM, Halvorsen OJ, Collett K, et al. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J Clin Oncol 2006;24:268–273.
Collett K, Eide GE, Arnes J, et al. Expression of enhancer of zeste homologue 2 is significantly associated with increased tumor cell proliferation and is a marker of aggressive breast cancer. Clin Cancer Res 2006;12:1168–1174.
Song LB, Zeng MS, Liao WT, et al. Bmi-1 is a novel molecular marker of nasopharyngeal carcinoma progression and immortalizes primary human nasopharyngeal epithelial cells. Cancer Res 2006;66:6225–6232.
Glinsky GV, Berezovska O, Glinskii AB . Microarray analysis identifies a death-from-cancer signature predicting therapy failure in patients with multiple types of cancer. J Clin Invest 2005;115:1503–1521.
Kim JH, Yoon SY, Jeong SH, et al. Overexpression of Bmi-1 oncoprotein correlates with axillary lymph node metastases in invasive ductal breast cancer. Breast 2004;13:383–388.
Silva J, Garcia JM, Pena C, et al. Implication of polycomb members Bmi-1, Mel-18, and Hpc-2 in the regulation of p16INK4a, p14ARF, h-TERT, and c-Myc expression in primary breast carcinomas. Clin Cancer Res 2006;12:6929–6936.
Dimri GP, Martinez JL, Jacobs JJ, et al. The Bmi-1 oncogene induces telomerase activity and immortalizes human mammary epithelial cells. Cancer Res 2002;62:4736–4745.
Liu S, Dontu G, Mantle ID, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res 2006;66:6063–6071.
Vonlanthen S, Heighway J, Altermatt HJ, et al. The bmi-1 oncoprotein is differentially expressed in non-small cell lung cancer and correlates with INK4A-ARF locus expression. Br J Cancer 2001;84:1372–1376.
Breuer RH, Snijders PJ, Smit EF, et al. Increased expression of the EZH2 polycomb group gene in BMI-1-positive neoplastic cells during bronchial carcinogenesis. Neoplasia 2004;6:736–743.
Kim JH, Yoon SY, Kim CN, et al. The Bmi-1 oncoprotein is overexpressed in human colorectal cancer and correlates with the reduced p16INK4a/p14ARF proteins. Cancer Lett 2004;203:217–224.
Hemmati HD, Nakano I, Lazareff JA, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci USA 2003;100:15178–15183.
Leung C, Lingbeek M, Shakhova O, et al. Bmi1 is essential for cerebellar development and is overexpressed in human medulloblastomas. Nature 2004;428:337–341.
Straume O, Sviland L, Akslen LA . Loss of nuclear p16 protein expression correlates with increased tumor cell proliferation (Ki-67) and poor prognosis in patients with vertical growth phase melanoma. Clin Cancer Res 2000;6:1845–1853.
Straume O, Akslen LA . Alterations and prognostic significance of p16 and p53 protein expression in subgroups of cutaneous melanoma. Int J Cancer 1997;74:535–539.
Bachmann IM, Straume O, Akslen LA . Altered expression of cell cycle regulators Cyclin D1, p14, p16, CDK4 and Rb in nodular melanomas. Int J Oncol 2004;25:1559–1565.
Straume O, Akslen LA . Expression of vascular endothelial growth factor, its receptors (FLT-1, KDR) and TSP-1 related to microvessel density and patient outcome in vertical growth phase melanomas. Am J Pathol 2001;159:223–235.
Breslow A . Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg 1970;172:902–908.
Clark Jr WH, From L, Bernardino EA, et al. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res 1969;29:705–727.
Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 1998;4:844–847.
Nocito A, Bubendorf L, Maria Tinner E, et al. Microarrays of bladder cancer tissue are highly representative of proliferation index and histological grade. J Pathol 2001;194:349–357.
Straume O, Akslen LA . Importance of vascular phenotype by basic fibroblast growth factor, and influence of the angiogenic factors basic fibroblast growth factor/fibroblast growth factor receptor-1 and ephrin-A1/EphA2 on melanoma progression. Am J Pathol 2002;160:1009–1019.
Alkema MJ, Bronk M, Verhoeven E, et al. Identification of Bmi1-interacting proteins as constituents of a multimeric mammalian polycomb complex. Genes Dev 1997;11:226–240.
Gunster MJ, Satijn DP, Hamer KM, et al. Identification and characterization of interactions between the vertebrate polycomb-group protein BMI1 and human homologs of polyhomeotic. Mol Cell Biol 1997;17:2326–2335.
Bracken AP, Dietrich N, Pasini D, et al. Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev 2006;20:1123–1136.
Kirmizis A, Bartley SM, Farnham PJ . Identification of the polycomb group protein SU(Z)12 as a potential molecular target for human cancer therapy. Mol Cancer Ther 2003;2:113–121.
Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature 2001;414:105–111.
Raaphorst FM, Meijer CJ, Fieret E, et al. Poorly differentiated breast carcinoma is associated with increased expression of the human polycomb group EZH2 gene. Neoplasia 2003;5:481–488.
Breuer RH, Snijders PJ, Sutedja GT, et al. Expression of the p16(INK4a) gene product, methylation of the p16(INK4a) promoter region and expression of the polycomb-group gene BMI-1 in squamous cell lung carcinoma and premalignant endobronchial lesions. Lung Cancer 2005;48:299–306.
Kang MK, Kim RH, Kim SJ, et al. Elevated Bmi-1 expression is associated with dysplastic cell transformation during oral carcinogenesis and is required for cancer cell replication and survival. Br J Cancer 2007;96:126–133.
Hsu MY, Meier FE, Nesbit M, et al. E-cadherin expression in melanoma cells restores keratinocyte-mediated growth control and down-regulates expression of invasion-related adhesion receptors. Am J Pathol 2000;156:1515–1525.
Li G, Satyamoorthy K, Herlyn M . N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer Res 2001;61:3819–3825.
Bachmann IM, Straume O, Puntervoll HE, et al. Importance of P-cadherin, beta-catenin, and Wnt5a/frizzled for progression of melanocytic tumors and prognosis in cutaneous melanoma. Clin Cancer Res 2005;11:8606–8614.
Bachmann IM, Straume O, Naumov GN, et al. Presence of tumor necrosis and apoptotic activity is associated with alterations of the molecular phenotype and clinical progress of cutaneous melanoma. 2006, submitted.
Integrin signalling during tumour progression. Nat Rev Mol Cell Biol 2004;5:816–826.
Acknowledgements
We thank Gerd Lillian Hallseth, Reidun Hægland, Bendik Nordanger and Randi Nygaard for excellent technical assistance. Grant support for this study was provided by Norwegian Cancer Society, Norwegian Research Council, Helse Vest HF.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure
The authors have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Bachmann, I., Puntervoll, H., Otte, A. et al. Loss of BMI-1 expression is associated with clinical progress of malignant melanoma. Mod Pathol 21, 583–590 (2008). https://doi.org/10.1038/modpathol.2008.17
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2008.17
Keywords
This article is cited by
-
Evaluation of the expression of Bmi-1 stem cell marker in sinonasal melanomas and its correlation with the expression of cell cycle proteins
Surgical and Experimental Pathology (2019)
-
Cartilage oligomeric matrix protein is a prognostic factor and biomarker of colon cancer and promotes cell proliferation by activating the Akt pathway
Journal of Cancer Research and Clinical Oncology (2018)
-
Differential BMI1, TWIST1, SNAI2 mRNA expression pattern correlation with malignancy type in a spectrum of common cutaneous malignancies: basal cell carcinoma, squamous cell carcinoma, and melanoma
Clinical and Translational Oncology (2017)
-
Tissue prognostic biomarkers in primary cutaneous melanoma
Virchows Archiv (2014)
-
BMI1 expression identifies subtypes of Merkel cell carcinoma
Virchows Archiv (2012)