Abstract
CD1d-restricted invariant natural killer T (iNKT) cells are known as potent early regulatory cells of immune responses. Besides the established roles in the regulation of inflammation and autoimmune disease, studies have shown that iNKT cells have important roles in tumor surveillance and the control of tumor metastasis. Here we found that the absence of iNKT cells markedly decreased the total number of intestinal polyps in APCMin/+ mice, a model for colorectal cancer. Polyp iNKT cells were enriched for interleukin-10 (IL-10)- and IL-17-producing cells, showed a distinct phenotype being CD4+, NK1.1− CD44int, and PD-1lo, and they were negative for the NKT cell transcription factor promyelocytic leukemia zinc-finger. The absence of iNKT cells was associated with a reduced frequency of regulatory T (Tregs) cells and lower expression levels of FoxP3 protein and transcript uniquely in the polyps, and a switch to an inflammatory macrophage phenotype. Moreover, in iNKT cell-deficient APCMin/+ mice, expression of T-helper (TH) 1-associated genes, such as IFN-γ and Nos2, was increased in polyps, concomitantly with elevated frequencies of conventional CD4+ and CD8+ T cells in this tissue. The results suggest that a population of regulatory iNKT cells locally promote intestinal polyp formation by enhancing Treg cells and immunosuppression of antitumor TH1 immunity.
Similar content being viewed by others
Introduction
Lipid-reactive CD1d-restricted natural killer T (NKT) cells are potent early regulatory cells of diverse immune responses. Besides the established roles in the regulation of immunity to infection, and in inflammation and autoimmune disease, many studies have attributed NKT cells an important role in tumor surveillance and the control of tumor metastasis.1 NKT cells can be divided into two functionally distinct categories according to their expression of T-cell receptors (TCRs).2, 3 An evolutionarily conserved category of NKT cells (termed invariant, iNKT cells, or type 1 NKT cells) expresses a semi-invariant TCR containing a specific TCRα chain (in humans Vα24-JαQ, in mice Vα14-Jα18).4 CD1d presents glycolipids to NKT cells, and essentially all iNKT cells are activated by the artificial lipid ligand α-galactosylceramide (αGalCer), first described for its strong antitumor effect.5 The remaining CD1d-reactive TCRαβ cells have diverse TCR (termed diverse, dNKT cells, or type 2 NKT cells).2 Studies using αGalCer treatment have established that iNKT cells have antitumor functions in several murine experimental models. Moreover, in some models iNKT cells demonstrate “natural” tumor surveillance, i.e., in the absence of activation with administered iNKT cell ligands.6, 7, 8 Interferon-γ (IFN-γ) production by iNKT cells has been identified as an essential component of their antitumor activity. Further, several reports found that cancer patients have reduced levels of NKT cells that show a functional deficiency in IFN-γ production, and that higher levels of NKT cells correlated with improved prognosis.9 On the other hand, dNKT cells have been found to suppress CD8+ T-cell-mediated tumor immunity in an interleukin-13 (IL-13) and tumor growth factor-β-dependent manner.9 Findings in peripheral blood from myeloma patients seem to support this dichotomy. An expanded population of dNKT cells was demonstrated, and interestingly, these cells produced IL-13 and were reactive to an inflammation-associated lysophospholipid presented on CD1d.10 Thus, different roles have often been associated with the two NKT subsets, and a model has been put forward proposing that iNKT cells promote tumor immunity, whereas dNKT cells suppress tumor immunity.9
Human inflammatory bowel disease increases the risk of developing colorectal cancer; consistent with a role for chronic inflammation in promoting carcinogenesis.11 In this context, it is significant that NKT cells were demonstrated to promote intestinal inflammation in Crohn’s disease and mouse models.12, 13, 14 The demonstrated role of NKT cells in chronic intestinal inflammation raises the question of whether these enhance inflammation-driven intestinal tumor formation by their capacity to promote inflammation, or prevent tumor formation at this site through their ability to provide tumor surveillance and tumor prevention. ApcMin/+ mice are a model for human colorectal cancer (CRC),15 which is the leading cause of cancer-related mortality worldwide. The ApcMin/+ mice have a truncated adenomatous polyposis coli (Apc) gene, a mutation that leads to the spontaneous formation of benign intestinal adenomatous polyps in heterozygote mice, recapitulating early events in human colorectal carcinogenesis. Several studies have demonstrated the critical role of inflammation driving the formation of polyps in human CRC and mouse models of the disease,16 and blocking of both innate and adaptive immune signals prevents tumor formation in ApcMin/+ mice.17, 18 Here, we have used the ApcMin/+ mouse model to determine the role of CD1d-restricted NKT cells in the regulation of polyp formation in the intestine.
Results
iNKT cells naturally promoted intestinal tumor development
We first determined whether the heterozygous Apc mutation in ApcMin/+ mice affected the numbers and the functions of iNKT cells. In our animal facility, 10–12-week-old ApcMin/+ mice had no macroscopically visible intestinal polyps, but early polyp formation could be seen on sections using a microscope (Figure 1a). iNKT cell frequencies were similar in the spleen, mesenteric lymph nodes (MLNs) (Figure 1b) and liver (data not shown) of ApcMin/+ and Apc+/+ mice at this age. Further, ApcMin/+ and Apc+/+ mice responded to αGalCer stimulation with vigorous production of cytokines at comparable levels detected in the serum at 2 to 24 h (Figure 1c). Thus, iNKT cells in 10–12-week-old ApcMin/+ mice were present in equal frequencies and demonstrated a normal in vivo responsiveness to αGalCer when compared with their Apc+/+ littermate control mice.
ApcMin/+ mice lacking invariant natural killer T (iNKT) cells had a decreased number of intestinal tumors. (a) Small intestinal lesion in 12-week-old ApcMin/+ mouse. Tissue sections were stained with hematoxylin/eosin; right panel shows the close-up of boxed part from left panel. (b) iNKT cells (TCR+αGalCer (PBS57)-loaded CD1d tetramer+ cells) in Apc+/+ and ApcMin/+ littermate mice were analyzed by flow cytometry. Symbols represent values from individual mice, mean±s.d. is indicated (n=7). (c) αGalCer was injected into ApcMin/+ and Apc+/+ mice, and serum cytokines were analyzed before (0 h) and at 2, 8, and 24 h after injection. Data (mean±s.d., n=3–5) are from one of two similar independent experiments. (d) Spleen weight, and (e) polyp numbers in the small intestine (SI) and colon in 15-week-old ApcMin/+Ja18−/− mice lacking iNKT cells compared with heterozygote control littermate ApcMin/+ Jα18+/− mice. (f) Development of intestinal polyps in 20-week-old ApcMin/+ CD1d−/− mice lacking CD1d compared with heterozygote control littermate ApcMin/+ CD1d+/− mice. Symbols represent individual mice, and median±s.d. is indicated in (d and f) 15 and (e) 25 mice. (g) Polyp numbers in ApcMin/+ mice treated from 5 weeks of age with αGalCer (C26:0) or the T-helper 2 (TH2) cytokine skewing analog C20:2 and killed at 15 weeks of age. Data are presented as median±s.d. (n=10). (b–f) Mann–Whitney test and (g) two-way analysis of variance (ANOVA) comparison with Bonferroni post-tests were used for statistical analyses. *P<0.05, **P<0.01, and ***P<0.001. TCR, T-cell receptor.
At 15 weeks of age, intestinal polyps were visible in all ApcMin/+ mice with a median of ∼20 polyps over the entire length of the intestine, with no significant difference between male and female ApcMin/+ mice (data not shown). To investigate the influence of iNKT cells on the natural course of polyp development in ApcMin/+ mice, we crossed the mice with Jα18−/− mice that specifically lack iNKT cells. The splenomegaly19 was reduced in ApcMin/+Jα18−/− mice compared with the ApcMin/+Jα18+/− controls (Figure 1d), and ApcMin/+Jα18−/− had an ∼75% reduction in the median number of polyps in the small intestine (SI) compared with the heterozygote (ApcMin/+Jα18+/−) littermate controls (Figure 1e). We also crossed ApcMin/+ mice with CD1d-deficient mice lacking all NKT cells. Compared with ApcMin/+CD1d+/− littermate controls, ApcMin/+CD1d−/− mice demonstrated a 56% decrease in SI polyp counts (Figure 1f). Neither of the two NKT-deficient mice harboring the ApcMin/+ mutation had significantly reduced numbers of polyps in the colon. Thus, two different mutations resulting in iNKT cell deficiency had reduced polyp numbers, demonstrating that iNKT cells naturally promote tumor development in this model. Moreover, the similar reduction in polyp numbers in mice lacking iNKT cells and all NKT cells suggests that dNKT cells do not have significant effects on tumor development in this model.
We next investigated the effect of activation of iNKT cells during polyp development. Mice were treated from 5 to 15 weeks of age with αGalCer that induces a mixed T-helper (TH)1/TH2 cytokine profile, or with the modified ligand C20:2 that induces preferential TH2 cytokine production by iNKT cells.20 αGalCer treatment reduced polyp numbers in both SI and colon, whereas SI polyp numbers in mice treated with C20:2 were significantly higher (Figure 1g). This demonstrated that ligand-activated iNKT cells have the capacity to control polyp development in both SI and colon, and indicated that activation of TH1 cytokine secretion by iNKT cells led to suppressed polyp development, whereas induction of iNKT cell-derived TH2 cytokines rather enhanced polyp development.
Unique phenotype and functions of iNKT cells in polyps of ApcMin/+ mice
To address the underlying mechanisms for the promotion of polyps in ApcMin/+ mice by iNKT cells, we first performed a broad analysis of iNKT cells in polyps and different lymphoid organs. Polyp-infiltrating lymphocytes contained ∼0.6% iNKT cells, which is similar to iNKT cell percentages in lamina propria (LP) lymphocytes from ApcMin/+ and Apc+/+ mice (Figure 2b). In MLN and spleen the frequencies of iNKT cells were comparable in both mice (Figure 2b). Owing to the splenomegaly of ApcMin/+ mice, the absolute number of splenic iNKT cells was slightly but significantly increased, while in contrast, the total numbers of CD4 and CD8 T and B cells remained the same (data not shown). In vitro stimulation of splenocytes induced similar frequencies in both mice of IL-4- and IFN-γ-producing iNKT cells (data not shown).
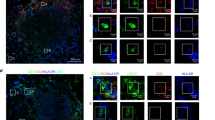
Polyp invariant natural killer T (iNKT) cells in ApcMin/+ mice displayed unique phenotype and functions, and lacked promyelocytic leukemia zinc-finger (PLZF) expression. iNKT cells were identified in 15-week-old mice using αGalCer (PBS57)-loaded CD1d-tetramers and anti-T-cell receptor-β (TCRβ) and gated as shown in (a). (b) Frequencies of iNKT cells in 15-week-old ApcMin/+ and Apc+/+ mice. (c) Representative stainings of CD4, NK1.1, CD44, and PD-1 expression on iNKT cells from the polyp and spleen of ApcMin/+ mice, and summary plots of marker expression by iNKT cells in different tissues of ApcMin/+ and Apc+/+ mice. Data are presented as mean±s.d. of 10 mice. (d) iNKT cells were defined as iNKT1, iNKT2, and iNKT17 cells according to the expression of PLZF, T-bet, and RAR-related orphan receptor γt (RORγt) as presented by the gates in the fluorescence-activated cell sorting (FACS) plots. Representative stainings of iNKT cells from ApcMin/+ mice are shown. Bar graphs in (d) show summary plots of the proportions of iNKT1, iNKT2, iNKT17, and iNKT-Pneg (PLZF-negative iNKT) cells in the lamina propria (LP) and polyp, mesenteric lymph node (MLN), and spleen of Apc+/+ and ApcMin/+ mice. (e) Summary plot of PLZF and T-bet staining median fluorescence intensity (MFI) in different organs. The levels of PLZF and T-bet in splenic conventional CD4 (Conv. CD4+) T cells are shown for comparison. (f) Representative staining of RORγt in iNKT cells and summary plot of the frequencies of RORγt+ iNKT cells in different organs. (d–f) Data from at least three independent experiments have been pooled and are presented as mean±s.d. of three to five mice. (g and h) Cytokine production by iNKT cells after 2 and 4 h of in vivo αGalCer stimulation. Data derive from two independent experiments, and six to eight mice per group. Each data point represents an individual mouse (spleen) or pooled organs from two mice (LP and polyp). Flow cytometry plots in (h) show representative staining of interleukin-10 (IL-10) and IL-17 in iNKT cells 4 h after αGalCer injection. Data represent mean±s.d., and Mann–Whitney test was used for statistical analyses. *P<0.05, **P<0.01, and ***P<0.001.
In an extended phenotypic analysis, we did not detect differences between iNKT cells when comparing the same tissues from ApcMin/+ and Apc+/+ mice, but we confirmed previous publications showing that iNKT cells at different locations have distinct phenotypes21 (Figure 2c). In polyps of ApcMin/+ mice, the fraction of iNKT cells expressing CD4 and NK1.1 was lower than in other organs. In contrast, CD69 (not shown) and CD44 was expressed by almost all polyp iNKT cells, but CD44 levels were strongly decreased compared with splenic iNKT cells, and only ∼10% of iNKT cells in polyps displayed the marker of activation/exhaustion, PD-1 (CD279) (Figure 2c). Similarly, LP iNKT cells demonstrated low proportions of cells displaying NK1.1 and high CD69; however, the majority of LP iNKT cells expressed PD-1. The iNKT population in MLN of both mice had similarly low frequencies of NK1.1+, CD69+, and PD-1+ cells (∼20%). Therefore, polyp iNKT cells displayed a cell surface phenotype that was different from iNKT cells in the other organs analyzed.
Expression of the transcription factor promyelocytic leukemia zinc-finger (PLZF) is a known feature of both developing and mature iNKT cells.22 In addition, iNKT cells can be divided into distinct functional subsets that express characteristic sets of transcription factors determining the pattern of cytokines secreted upon activation,23 analogous to T-helper 1 (TH1), TH2, and TH17 cells. Three major functional subsets have been defined, iNKT1, iNKT2, and iNKT17, that are distributed in different ratios in various lymphoid organs. We stained polyp iNKT cells for intracellular transcription factors and compared them with iNKT cells in LP, MLN, and spleen (Figure 2d–f). Surprisingly, polyp iNKT cells were essentially negative for PLZF, with a similar median fluorescence intensity (MFI) level to splenic CD4+ T cells. These PLZF-negative iNKT cells fall outside the established gating for iNKT1/2/17 cell subsets (see gating in Figure 2d). Here, we have termed the PLZF-negative iNKT cells “iNKT-Pneg”. Strikingly, over 90% of iNKT cells in polyps were iNKT-Pneg. iNKT cells in LP of both mice expressed PLZF, but at lower levels than iNKT cells from the spleen and MLN. Among iNKT cells in control Apc+/+ LP, ∼30% were iNKT-Pneg by our definition, and ∼55% were iNKT1, whereas LP of ApcMin/+ mice had slightly but significantly lower frequency of iNKT1 cells and a somewhat increased frequency of iNKT-Pneg cells. Splenic iNKT cells consisted almost exclusively of iNKT1 cells, whereas MLN iNKT cells displayed equal frequencies of iNKT1 and iNKT2 cells and a distinct iNKT17 population of ∼10%, and there was no difference between iNKT cells from ApcMin/+ and Apc+/+ mice in these organs. Around 10% of all iNKT cells in MLN, LP, and polyp expressed RAR-related orphan receptor γt (RORγt) (Figure 2f), whereas the levels of RORγt were somewhat lower in polyp iNKT cells compared with other organs. Some of the RORγt+ iNKT cells in LP, and the majority in polyps, were PLZF negative (thereby falling within the iNKT-Pneg definition in Figure 2d). Thus, polyp iNKT cells displayed a transcription factor pattern that was different from the established iNKT functional subsets, most notably by lacking the NKT cell transcription factor PLZF. This suggests that polyp iNKT cells possess a distinct functional capacity that underpins tumor promotion occurring at this site.
Considering that iNKT cells in polyps did not express the expected transcription factors that determine iNKT cell functional status, we determined intracellular cytokines in iNKT cells activated in vivo with αGalCer (Figure 2g and h). As shown before,24 we found that MLN iNKT cells responded poorly to systemic αGalCer stimulation (data not shown). Unstimulated iNKT cells in polyps contained detectable frequencies of cells positive for IL-2, IL-4, IL-13, tumor necrosis factor-α, and IL-17 but not IFN-γ, something that was also seen in LP, but was absent in splenic iNKT cells. After in vivo stimulation, iNKT cells in polyps harbored significant frequencies of cells positive for IFN-γ (∼20%), IL-2, IL-4, IL-13, and tumor necrosis factor-α (all ∼10%); however, the frequencies were lower than found in splenic iNKT cells. Strikingly, however, polyp iNKT cells were strongly enriched for cells containing IL-10 or IL-17 compared with both spleen and LP iNKT cells, particularly evident at 4 h (Figure 2h). Taken together, this demonstrates that αGalCer-activated polyp iNKT cells secreted a different pattern of cytokines compared with spleen and LP iNKT cells, notably being enriched for IL-10- and IL-17-producing cells.
iNKT cells controlled accumulation, activation, and functional status of polyp-infiltrating T cells
We next defined differences in T cells infiltrating the polyp in iNKT cell-deficient ApcMin/+Jα18−/− and ApcMin/+Jα18+/− mice. Lack of iNKT cells resulted in elevated frequencies of CD8+ T cells in polyps as well as of conventional CD4+ T cells (CD4+ FoxP3−, convCD4 cells) in both polyps and MLN of ApcMin/+Jα18−/− mice (Figure 3a). Further, in the absence of iNKT cells the expression of PD-1 was reduced on CD8 T cells in both polyps and LP and on convCD4 T cells in LP, consistent with a lower degree of exhaustion (Figure 3b and c). In polyps, where PD-1 expression on convCD4 cells was very low, the frequency of PD-1+ cells was slightly increased. Moreover, in polyp, LP, and MLN there was a significant reduction in the expression of the IL-33 receptor ST2, associated with TH2 cells, by convCD4 cells (Figure 3c), suggesting that iNKT cells provide an environment that is more supportive of this TH subset. Both CD8 and convCD4 T populations displayed strongly reduced frequencies of CD69+ cells in polyps of ApcMin/+Jα18−/− mice compared with ApcMin/+Jα18+/− mice. CD69 is known as a marker of early activation of T cells in lymphoid organs and tissue-resident memory T cells; however, studies also suggest that CD69 negatively regulates proinflammatory functions of T cells in tissues (see Gonzalez-Amaro et al.25 for review). In the absence of iNKT cells, a lower frequency of convCD4 and CD8 T cells within polyp tissue was engaged in proliferation, as indicated by reduced Ki67 expression. Taken together, this demonstrates that, directly or indirectly, iNKT cells control accumulation and activation/functional status of convCD4 and CD8 T cells in the polyp tissue.
Increased frequency of conventional T cells in polyps in the absence of invariant natural killer T (iNKT) cells. (a) Frequency of conventional CD4+ (CD4+FoxP3−) cells and CD8+ T cells among lymphocytes in ApcMin/+ Jα18−/− mice and their ApcMin/+ Jα18+/− littermate controls. (b) Representative staining and summary data of CD69, Ki67, and PD-1 expression on CD8+ T cells in ApcMin/+Ja18−/− and ApcMin/+ Jα18+/− mice. (c) Representative staining and summary data of ST2, CD69, Ki67, and PD-1 expression on conventional CD4+ T cells in ApcMin/+Ja18−/− and ApcMin/+ Jα18+/− mice. Data from at least three independent experiments are presented as mean±s.d. of seven to ten mice (CD69) and three to six mice (Ki67, PD-1, and ST2) per genotype. Mann–Whitney test was used for statistical analyses. *P<0.05, **P<0.01, and ***P<0.001.
iNKT cells promoted a regulatory, and suppressed a Th1/proinflammatory polyp microenvironment
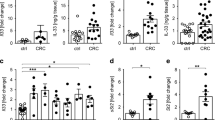
To determine the immune- and tumor-related molecular signatures that distinguish the iNKT cell-mediated promotion of tumor burden in ApcMin/+ mice, a quantitative PCR array screen was performed for the expression of a set of genes relevant for immune responses, tumor growth, and apoptosis (Figure 4a and b). We considered that iNKT cells may promote tumor development by proinflammatory actions increasing the production of tumor growth factors, and also the possibility that tumor enhancement was due to increased immunoregulation, resulting in decreased tumor immunity. Gene expression in polyp and LP from ApcMin/+Jα18−/− and ApcMin/+Jα18+/− mice were compared, resulting in the identification of several genes that were altered in expression in the absence of iNKT cells (Figure 4a and b). Lack of iNKT cells resulted in upregulated expression in polyps of genes associated with a Th1/proinflammatory immune response, such as those encoding IFN-γ, iNOS (Nos2), IL12p40, T-bet (Tbx21), granzyme B (Gzmb), and Stat-1. In contrast, transcript levels of Foxp3, the master transcription factor for regulatory T (Treg) cells, in polyps were decreased. Interestingly, iNKT cell deficiency resulted in enhanced Th1/inflammation-associated gene expression also in LP (increased expression of genes encoding iNOS, IFN-γ, CD8β, CXCL9, CXCL10, granzyme B). We performed quantitative PCR on tissue samples from individual mice for a set of genes to verify the array results (Figure 4c). The results confirmed significantly higher expression levels of Ifng and Nos2 in both polyp and LP in the absence of iNKT cells. In iNKT-sufficient control mice, the levels of both Il17a and Foxp3 transcripts were higher in polyp tissue compared with LP. Notably, the absence of iNKT cells was associated with a decrease in Foxp3 expression in the polyps but not LP. This strongly indicates that iNKT cells promoted polyp development by enhancing immune regulation and suppressing Th1-associated tumor immunity.
Absence of invariant natural killer T (iNKT) cells promoted expression of inflammatory genes and reduced immunoregulatory genes in polyp tissue. The expression of mRNA from unaffected lamina propria (LP) and polyp tissue of 15-week-old ApcMin/+ Jα18−/− and ApcMin/+ Jα18+/− mice was examined by RT2 PCR profiler array with a selection of genes of relevance for (a and b) immunity and tumor progression (each sample was a pool of mRNA from five mice) and (c) real-time PCR. (a) Heat map of gene expression. (b) Normalized expression values for polyp (upper) and LP (lower) tissues. ApcMin/+ Jα18−/− “up” (red) and “down” (green) signature transcripts are highlighted. The central line indicates unchanged gene expression, and the boundary lines indicate fourfold regulation. (c) The expression of selected genes were examined by real-time PCR and normalized against β-actin. Symbols represent individual mice, and data are presented as mean±s.d. of five mice. Mann–Whitney test was used for statistical analyses. *P<0.05, **P<0.01, and ***P<0.001.
iNKT cells supported the expression of FoxP3 and an activated Treg cell phenotype in polyps
The decreased FoxP3 expression in polyps from ApcMin/+ Jα18−/− mice compared with ApcMin/+Jα18+/− mice prompted a comprehensive analysis of Treg cells by flow cytometry (Figure 5). In iNKT cell-deficient polyps, but not LP or other lymphoid organs, the frequency of Treg cells (both when gated as CD25+ FoxP3+ cells or as FoxP3+ cells) among CD4+ cells was significantly decreased (Figure 5a and b). Notably, also the MFI level of FoxP3 in CD25+ CD4+ T cells was reduced in ApcMin/+Jα18−/− mice. The same was true when FoxP3 levels were compared within the CD25+ FoxP3+ gate, suggesting that iNKT cells promote Treg in a manner that maintains higher levels of FoxP3 protein. Further, iNKT cells reinforced expression of the IL-33 receptor ST2 on both polyp and LP Treg cells. Extended phenotypic analysis revealed that the presence of iNKT cells was associated with an increased fraction of polyp Treg cells expressing CD69 (Figure 5c), and of LP Treg cells expressing PD-1, whereas KLRG1 expression was not influenced by iNKT cells (not shown). Taken together, this demonstrates that iNKT cells drive Treg cell maintenance and/or activation both in polyps and LP of ApcMin/+ mice.
Invariant natural killer T (iNKT) cells supported the expression of FoxP3 and an activated polyp regulatory T (Treg) cell phenotype. (a) Representative stainings show a decreased level of FoxP3 protein in Treg cells from ApcMin/+Ja18−/− and ApcMin/+ Jα18+/− mice. (b) Frequencies of CD25+FoxP3+ T cells (left) and total FoxP3+ T cells (right) among CD4+ T cells in ApcMin/+Ja18−/− and ApcMin/+ Jα18+/− mice. (c) Median fluorescence intensity (MFI) of FoxP3 staining in CD25+ cells (left) or in FoxP3+ cells (right) in different tissue in the presence and absence of iNKT cells. (d) Representative staining of CD69 and ST2 on Treg cells in polyps from ApcMin/+Ja18−/− mice and ApcMin/+ Jα18+/− mice. (e) Surface expression of CD69, PD-1, KLRG1, and ST2 on Treg cells in ApcMin/+Ja18−/− mice and their ApcMin/+ Jα18+/− littermates. Data are presented as mean±s.d. of seven mice from (a and b) three independent experiments and (c) three mice from a representative experiment of three performed, and (e) three mice from two independent experiments. Mann–Whitney test was used for statistical analyses. *P<0.05, **P<0.01, and ***P<0.001.
Absence of iNKT cells led to a systemic shift in macrophage phenotype from M2 to M1
As we found that Nos2, expressed by macrophage subtype 1 (M1) cells, was the most strongly upregulated gene in ApcMin/+Jα18−/− polyp and LP compared with ApcMin/+Jα18+/− tissues, we investigated innate immune cells in polyps, LP, and spleen (Figure 6). MLN contained very low frequencies of these cells (data not shown). In the presence of iNKT cells there was an elevated frequency of CD11chi dendritic cells in the polyps compared with iNKT cell-deficient mice (Figure 6a and b). There was also a several-fold higher proportion of cells with a phenotype of myeloid-derived suppressor cells (MDSCs) commonly found in tumors26. Further, there was a significant increase in the frequency of F4/80+ macrophages in the spleen (Figure 6a and b), consistent with the splenomegaly in ApcMin/+Jα18+/− mice (Figure 1d). We next determined the macrophage expression of iNOS and CD206, characteristic of M1 and M2 macrophages, respectively. Strikingly, iNKT cell deficiency resulted in a highly significant shift in the macrophage phenotype in all tissues, from a mixed/M2-dominated population (high frequency of CD206+/low frequency of iNOS+ cells) in ApcMin/+Jα18+/− mice to a macrophage population strongly skewed toward the M1 phenotype in ApcMin/+Jα18−/− mice (high frequency of iNOS+ and strongly diminished proportion of CD206+ macrophages) (Figure 6c and d). In the spleen, the proportion of macrophages expressing CD11c, also described as an M1 macrophage marker, was increased in the absence of iNKT cells, whereas, surprisingly, in polyps of the same mice the frequency of macrophages expressing CD11c was reduced (Figure 6d). Interestingly, in polyps and other tissues analyzed we found that CD11c and iNOS were expressed by distinct subsets of F4/80+ cells (data not shown), indicating heterogeneity of macrophages beyond a simple M1/M2 division. Further analysis of the MDSC population revealed that the majority of MDSC in iNKT-sufficient ApcMin/+ mice were polymorphonuclear-MDSC (PMN-MDSC; Ly6Cint/Ly6G+). This population was strongly reduced in ApcMin/+Jα18−/− mice lacking iNKT cells and similar in frequencies to mononuclear MDSC (Ly6Chi/Ly6G−) in these mice (Figure 6e and f). Thus, in the absence of iNKT cells, there was a strong shift from CD206+ M2 toward iNOS+ M1 macrophages in all tissues analyzed, and a reduction of PMN-MDSC most significantly in polyps.
Lack of invariant natural killer T (iNKT) cells resulted in a skewed macrophage phenotype from M2 to M1. Innate immune cells were investigated by flow cytometry in polyps, lamina propria (LP), and spleen of ApcMin/+ Jα18−/− and ApcMin/+ Jα18+/− mice. (a) Gating strategies for dendritic cells (DCs, CD3−CD19−CD45+CD11chi), myeloid-derived suppressor cells (MDSCs) (CD3−CD19−CD45+CD11clo/negCD11b+), and macrophages (Mø, CD3−CD19−CD45+CD11clo/negF4/80+) are shown for ApcMin/+ Jα18+/− polyp, LP, and spleen. (b) Frequency of DCs, MDSCs, and Mø among CD45+ cells in indicated tissues. (c) Representative staining of iNOS and CD206 expression on macrophages. (d) Frequency of macrophages expressing iNOS, CD206, and CD11c in the same tissues. Data from three independent experiments are presented as mean±s.d. of six mice. (e) Gating strategy for mononuclear MDSC (M-MDSC) (CD3−CD19−CD45+CD11clo/negCD11b+Ly6ChiLy6G−) and polymorphonuclear-MDSC (PMN-MDSC) (CD3−CD19−CD45+CD11clo/negCD11b+Ly6CintLy6G+). (f) Frequency of M-MDSC and PMN-MDSC in ApcMin/+ Jα18−/− and ApcMin/+ Jα18+/− mice. (g) ApcMin/+ Jα18−/− mice were adoptively transferred with 5 × 105 hepatic iNKT cells or CD1d tetramer-negative αβ T cells (αβT) twice, at 13 and 14.5 weeks of age and killed at 16 weeks of age. Nontransferred mice were also used as control. Representative stainings of iNOS and CD206 expression on macrophages are shown for each group. (h) Frequency of macrophages expressing iNOS and CD206 in the same tissues. Data are presented as mean±s.d. of four mice. Mann–Whitney test was used for statistical analyses. *P<0.05, **P<0.01, and ***P<0.001.
To reinforce the notion that iNKT cells regulate the inflammatory microenvironment in polyps, we transferred sorted iNKT cells to ApcMin/+Jα18−/− mice and 3 weeks later investigated the effects of the transfer on macrophage phenotypes. Transfer of iNKT cells resulted in a striking reduction of iNOS expression and increase in CD206 expression among macrophages in the polyps (Figure 6g and h). In contrast, there was no significant change in macrophage expression of these markers in LP or spleen after iNKT cell transfer. Transfer of iNKT cell-depleted T cells did not change the macrophage phenotype in any of the organs compared with nontransferred mice. This is consistent with a model in which iNKT cells promote an anti-inflammatory environment specifically in the polyps, resulting in the enhancement of M2 macrophages.
Discussion
iNKT cells are well known for their potent tumor immunosurveillance and the suppression of tumors after αGalCer therapy. In such models, activated iNKT cells combat tumors by the production of IFN-γ and downstream activation of cells including NK cells, dendritic cells and cytotoxic CD8 T cells, and by being directly cytotoxic against CD1d-expressing tumor cells (see for a review Terabe and Berzofsky27). In contrast, in the present study, using two different mutant mouse strains that lack iNKT cells, we demonstrate that iNKT cells promoted the development of spontaneous intestinal polyps in ApcMin/+ mice, a model for early stages of human colorectal cancer. Surprisingly, we found that iNKT cell promotion of polyps was associated with an immunoregulatory polyp microenvironment, characterized by increased Treg and PMN-MDSC populations and an enhanced M2 macrophage phenotype, but suppressed TH1 immunity and reduced frequencies of tumor-infiltrating conventional CD4 and CD8 T cells. The results are summarized in the simplified model in Figure 7.
Invariant natural killer T (iNKT) cells suppressed T-helper 1 (TH1) immunity and promoted an immunoregulatory microenvironment in polyps. The findings are summarized in this proposed model for iNKT cells promotion of intestinal polyps.
The absence of iNKT cells in ApcMin/+ mice was associated with increased expression of IFN-γ and iNOS, and a systemic switch in macrophage phenotype from M2 to M1. These changes are likely to be pivotal for the reduced tumor burden in ApcMin/+Jα18−/− mice. First, ApcMin/+ mice lacking iNOS have significantly more adenomas than iNOS-positive littermate controls.28 This is in line with human studies showing that high infiltration of cells positive for iNOS correlated with significantly improved prognosis in CRC.29 Moreover, M2- or M2-like tumor-associated macrophages are known to support tumor progression and suppress tumor immunity, whereas M1 macrophages are involved in antitumor immunity.30 Second, IFN-γ is shown to be tumor suppressive in ApcMin/+ mice.31 The source of IFN-γ was not investigated in our studies, but CD8+ T cells are likely candidates. Consistently, we found that the frequency of infiltrating CD8 T cells, as well as CD4 T cells, was increased in the absence of iNKT cells. This may be an indirect effect and result from the reduction of Treg frequency and activation status in ApcMin/+Jα18−/− mice, as it has been shown that depletion of Treg in ApcMin/+ mice results in the influx of T cells into the polyps.32 Also, in some other murine tumor models, iNKT cells have been shown to promote tumor growth, associated with suppressed CD8 CTL, decreased IFN-γ, and elevated IL-13 production.33, 34 The underlying mechanism of suppression of tumor immunity by iNKT cells in these models may be similar to what has been described for dNKT cells in a series of studies by Terabe et al.35, 36 Here, IL-13 production by dNKT cells induced TGF-β production by CD11b+ Gr-1+ myeloid cells, which in turn inhibited the activation of tumor-specific CD8+ effector cells resulting in enhanced tumor growth. Such myeloid cells may be involved in the suppression of immunity in ApcMin/+ polyps as we found that the presence of iNKT cells also increased the polyp frequency of cells with a phenotype of MDSC that express Ly6G, a marker of PMN-MDSC that are strongly associated with tumor-promoting activity.26 Tumor infiltration by CD8 T cells has been associated with improved prognosis in many cancers including CRC,37 and more recently, extended studies of infiltrating immune cells in human CRC have established their important prognostic value, more broadly demonstrating that TH1 immunity and CD8 T cells in tumor infiltrates correlate with increased disease-free survival of patients.38 Thus, the suppression of TH1 immunity in ApcMin/+ polyps by the presence of iNKT cells is likely to be a major cause for the increased polyp numbers. In contrast, expression of IL-17A is associated with tumor promotion in human CRC and ApcMin/+ mice.18, 39 We found that IL-17A transcript levels were highly variable in polyps in the presence of iNKT cells, but not significantly altered in polyps in iNKT cell-deficient ApcMin/+ mice.
iNKT cell regulation of the immune environment in the polyps may partly be due to the promotion by iNKT cells of infiltrating Treg cells that had a phenotype associated with a higher activation state and elevated effector function. We show that in the presence of iNKT cells, the amount of FoxP3 mRNA in polyps was increased, and the frequency of positive cells and the fluorescence intensity of FoxP3 staining in Treg cells were augmented. Such differences were not detected in other tissues. Moreover, in the presence of iNKT cells, Treg cells in polyps showed a marked upregulation of ST2. ST2 was recently demonstrated to be expressed on high proportions of Treg cells in the colon as well as in the adipose tissue.40, 41, 42 IL-33 promoted their immunoregulatory function and maintenance of the Treg phenotype, including maintenance of FoxP3 expression, and increased expression of its own receptor ST2 on Treg. Importantly, ST2 deficiency protects from tumor development in an induced mouse model of CRC.43 Taken together, this suggests that iNKT cells promote the accumulation, activation and/or homeostasis of highly active immunoregulatory Treg cells in the polyp tissue. It is interesting to note that the induction of Treg cells by iNKT cells has also been shown to have a role for tolerance in autoimmune setting.s27
Although other tissues were also influenced, the most striking differences resulting from the absence of iNKT cells in ApcMin/+ mice were revealed in the polyps, especially with regard to Treg frequency and phenotype, suggesting that iNKT cells regulate immune cells in this tissue. This was also suggested by the polyp-specific effect on MDSC by depletion of iNKT cells in ApcMin/+ mice, and by the promotion of an M2 macrophage phenotype specifically in the polyps after transfer of iNKT cells to ApcMin/+Jα18−/− mice. We found that iNKT cells infiltrated the ApcMin/+ polyps, but they were present in a relatively low frequency at this site. Polyp iNKT cells were predominantly NK1.1−, CD44int, PD-1−, and around half of them were negative for CD4, which differed from iNKT cells in LP and lymphoid organs, and strikingly, they were negative for PLZF. Moreover, while polyp iNKT cells had a generally lower cytokine production in vivo in response to αGC, they demonstrated a highly increased capacity to make IL-10 and IL-17. These polyp iNKT cells share some functional features and cell surface phenotype with the so-called iNKT10 cells that arise after strong αGC stimulation in vivo,44 and with iNKT cells that are present in white adipose tissue.44, 45 Both adipose tissue iNKT and iNKT10 cells produced IL-10 upon activation, but had a decreased production of other tested cytokines compared with splenic iNKT cells in naive mice, and were ascribed regulatory functions. Similar to iNKT cells in ApcMin/+ mice, the induced iNKT10 cells suppressed tumor immunity and this effect was dependent on IL-10.44 Adipose tissue iNKT cells were shown to favor a noninflammatory adipose environment by promoting the homeostasis and regulatory function of adipose tissue Treg through the production of IL-2, and moreover, IL-10 dependently induced an anti-inflammatory state in local macrophages.45 Here, we demonstrate that polyp iNKT cells had very similar effects on polyp Treg cells and macrophages, despite the sevenfold lower frequency of iNKT cells in polyps (∼0.6%) compared with adipose tissue (∼4%; Lynch et al.45). The production of IL-10 and IL-2 by polyp iNKT cells may contribute to these effects. It was proposed that the adipose tissue regulatory iNKT phenotype was induced by chronic local stimulation. This may be true also for polyp iNKT cells, and is consistent with their low but constitutive production of some cytokines. A local instruction of the iNKT cell phenotype in polyps was also suggested by the fact that transfer of liver iNKT cells, which were predominantly iNKT1 (data not shown), to ApcMin/+Jα18−/− mice reconstituted the polyp macrophage skewing toward M2 similar to what was found in ApcMin/+Jα18+/− mice. A different scenario was proposed in a recent publication demonstrating that unique features of the TCR could determine the development of iNKT cells with a phenotype of adipose iNKT cells in the thymus, followed by subsequent accumulation of these iNKT cells in adipose tissue.46 It remains to be investigated to what extent the tissue microenvironment induces the polyp iNKT-Pneg or adipose tissue phenotype of iNKT cells, and which signals may be required for the development of these unusual iNKT cell phenotypes.
The fact that iNKT cells have been shown to be protective in several tumor models have led to early clinical trials that have shown some promising outcomes in cancer patients who have received iNKT cell-directed therapy.47 Our results reveal novel aspects on iNKT cell regulation of tumor immunity, and encourage more detailed studies of the relative contributions of iNKT cells to antitumor immunity and immunoregulation in tumor immunity to fully understand their role and underlying mechanisms at different stages, and to evaluate the prospect of iNKT cell-directed therapy in these diseases.
Methods
Mice. The ApcMin/+ mutation occurred and is maintained on the C57BL/6 genetic background.15 Sex- and age-matched mice were used in all experiments. ApcMin/+ mice were crossed with Jα18−/− mice to obtain ApcMin/+Jα18−/− and littermate control ApcMin/+Jα18+/− mice. ApcMin/+ mice were also crossed with CD1d−/− mice to obtain ApcMin/+CD1d−/− and littermate control ApcMin/+CD1d+/− mice. Both male and female mice from these crosses were used for experiments. All mice were bred and maintained at the department of Experimental Biomedicine, University of Gothenburg. Animal experiments in this study were approved by the animal ethics committee in Gothenburg.
Quantification of polyps. Mice were killed at 15 or 20 weeks of age. The intestines were flushed with phosphate-buffered saline (PBS) from both ends using blunt-end gavage needles to remove fecal material, and cut into three equal length segments: duodenum, jejunum, and ileum. Each segment was then cut open longitudinally and polyps were counted under stereomicroscope.
In vivo treatment with glycolipid. Mice were injected intraperitoneally with 4μg of glycolipids (α-GalCer C26:0, α-GalCer C20:2) in 200 μl of PBS with a final concentration 0.1% dimethyl sulfoxide and 0.05% Tween-20. Vehicle control was prepared and injected in an identical manner. The 5-week-old female ApcMin/+ mice were treated with glycolipids or vehicle control on day 1, 2, 7, 14, 21, 28, and 60, and the mice were killed at 15 weeks of age.
Histology. Tissue rolls of 12-week-old mouse intestine were fixed with formalin at room temperature for 24 h. After processing, the formalin-fixed, paraffin-embedded tissues were vertically sectioned lengthwise, and sections stained with hematoxylin and eosin (performed by Histo-Center AB, Västra Frölunda, Sweden).
Lymphocyte preparation. Spleen, MLN, and SI were collected from 15-week-old ApcMin/+ or ApcMin/+ Jα18−/− mice and their littermate control mice. Single-cell suspensions from the spleen and MLN were prepared. LP lymphocytes and tumor-infiltrating lymphocytes were isolated from the SI after removal of Peyer’s patches. The tissue was dissected into tumor and unaffected tissue to be processed separately. Unaffected tissue was cut into small pieces. Tumor and unaffected tissues were dissociated with Lamina Propria Dissociation Kit (Miltenyi Biotech, Bergisch Gladbach, Germany). Undigested tissue was removed by filtration and lymphocytes counted using trypan blue.
Flow cytometry. See Supplementary Material online.
In vivo stimulation with αGalCer and serum cytokine determination. αGalCer were first dissolved in 100% dimethyl sulfoxide, followed by a dilution with 0.5% Tween-PBS to achieve a stock concentration of 172.4 μg glycolipid per ml in 1% dimethyl sulfoxide and 0.5% Tween-PBS. This stock was stored at −20 °C. Upon use, the glycolipid stock was thawed and sonicated for 5 min and immediately heated at 80 °C for 2 min in glass vials. After vortexing for 1 min, the required volume of glycolipid stock was dissolved in prewarmed (37 °C) PBS and kept in an 80 °C bath until shortly before injection. Each mouse was injected intraperitoneally with 4 μg of αGalCer in 200 μl of PBS with a final concentration 0.1% dimethyl sulfoxide 0.05% Tween-20. Mice were bled before αGC injection, and at different times (2, 8, and 24 h respectively) after injection. Serum was prepared by centrifugation and stored at −20 °C. Cytokine content was measured using a CBA Mouse Th1/Th2/Th17 Cytokine Kit (BD Bioscience, Franklin Lakes, NJ) and the samples were analyzed with a LSRII Flow Cytometer (BD Bioscience).
RNA isolation, cDNA synthesis and quantitative PCR. See Supplementary Material.
Quantitative RT2 profiler PCR arrays. The 384 (4 × 96)-well custom-made PCR array (SA Bioscience, Germantown, MD) layout contained four replicate primer assays for each of 86 target genes and 7 housekeeping genes. In addition, 4 wells contained mouse genomic DNA controls, and 12 wells contained positive PCR controls. Prepared cDNA from pools of five mice were added (for polyps in duplicates) to RT2 SYBR Green Mastermix and the mix was aliquoted into the PCR array plates. PCR reactions were performed by the Genomics Core Facility, The Sahlgrenska Academy, and relative expression levels were determined using data from the real-time cycler and the ΔΔCT method.
iNKT cell isolation and adoptive transfer. Hepatic mononuclear cells (for hepatic mononuclear cell isolation see Supplementary Material) were stained with TCRβ and PBS57-loaded CD1d tetramer-PE and sorted with FACSAria II (BD). CD1d tetramer+ iNKT cells (5 × 105) or CD1d tetramer-negative TCRβ+ cells were injected intravenously into 13- and 14.5-week-old ApcMin/+Jα18−/− mice. Nontransferred mice were used as control. Mice were killed 3 weeks after the first injection. Lymphocytes from spleen, MLN, polyps, and unaffected lamina propria were isolated and analyzed with flow cytometry.
Statistical analysis. Calculation of statistical significance was performed using nonparametric Mann–Whitney test or two-way analysis of variance comparison with Bonferroni post-tests. P values of <0.05 were considered significant. Statistical analyses were performed on Prism GraphPad 6 (GraphPad Software, La Jolla, CA). Results are presented as mean±s.d. in the figures.
References
Smyth, M.J., Crowe, N.Y., Hayakawa, Y., Takeda, K., Yagita, H. & Godfrey, D.I. NKT cells—conductors of tumor immunity? Curr. Opin. Immunol. 14, 165–171 (2002).
Cardell, S., Tangri, S., Chan, S., Kronenberg, M., Benoist, C. & Mathis, D. CD1-restricted CD4+ T cells in major histocompatibility complex class II-deficient mice. J. Exp. Med. 182, 993–1004 (1995).
Stenstrom, M. et al. Surface receptors identify mouse NK1.1+ T cell subsets distinguished by function and T cell receptor type. Eur. J. Immunol. 34, 56–65 (2004).
Bendelac, A., Lantz, O., Quimby, M.E., Yewdell, J.W., Bennink, J.R. & Brutkiewicz, R.R. CD1 recognition by mouse NK1+ T lymphocytes. Science 268, 863–865 (1995).
Kitamura, H. et al. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J. Exp. Med. 189, 1121–1128 (1999).
Bellone, M. et al. iNKT cells control mouse spontaneous carcinoma independently of tumor-specific cytotoxic T cells. PLoS ONE 5, e8646 (2010).
Smyth, M.J. et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J. Exp. Med. 191, 661–668 (2000).
Swann, J.B. et al. Type I natural killer T cells suppress tumors caused by p53 loss in mice. Blood 113, 6382–6385 (2009).
Robertson, F.C., Berzofsky, J.A. & Terabe, M. NKT cell networks in the regulation of tumor immunity. Front. Immunol. 5, 543 (2014).
Chang, D.H. et al. Inflammation-associated lysophospholipids as ligands for CD1d-restricted T cells in human cancer. Blood 112, 1308–1316 (2008).
Ullman, T.A. & Itzkowitz, S.H. Intestinal inflammation and cancer. Gastroenterology 140, 1807–1816 (2011).
Heller, F., Fuss, I.J., Nieuwenhuis, E.E., Blumberg, R.S. & Strober, W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity 17, 629–638 (2002).
Fuss, I.J. et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J. Clin. Invest. 113, 1490–1497 (2004).
Liao, C.M., Zimmer, M.I., Shanmuganad, S., Yu, H.T., Cardell, S.L. & Wang, C.R. dysregulation of CD1d-restricted type II natural killer T cells leads to spontaneous development of colitis in mice. Gastroenterology 142, 326–334 e321–e322 (2012).
Moser, A.R., Pitot, H.C. & Dove, W.F. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 247, 322–324 (1990).
Monteleone, G., Pallone, F. & Stolfi, C. The dual role of inflammation in colon carcinogenesis. Int. J. Mol. Sci. 13, 11071–11084 (2012).
Rakoff-Nahoum, S. & Medzhitov, R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science (New York, NY) 317, 124–127 (2007).
Chae, W.J., Gibson, T.F., Zelterman, D., Hao, L., Henegariu, O. & Bothwell, A.L. Ablation of IL-17A abrogates progression of spontaneous intestinal tumorigenesis. Proc. Natl Acad. Sci. USA 107, 5540–5544 (2010).
You, S. et al. Developmental abnormalities in multiple proliferative tissues of Apc(Min/+) mice. Int. J. Exp. Pathol. 87, 227–236 (2006).
Carreno, L.J., Saavedra-Avila, N.A. & Porcelli, S.A. Synthetic glycolipid activators of natural killer T cells as immunotherapeutic agents. Clin. Transl. Immunol. 5, e69 (2016).
Kim, E.Y., Lynch, L., Brennan, P.J., Cohen, N.R. & Brenner, M.B. The transcriptional programs of iNKT cells. Semin. Immunol. 27, 26–32 (2015).
Savage, A.K. et al. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity 29, 391–403 (2008).
Lee, Y.J., Holzapfel, K.L., Zhu, J., Jameson, S.C. & Hogquist, K.A. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat. Immunol. 14, 1146–1154 (2013).
Lee, Y.J., Wang, H., Starrett, G.J., Phuong, V., Jameson, S.C. & Hogquist, K.A. Tissue-specific distribution of iNKT cells impacts their cytokine response. Immunity 43, 566–578 (2015).
Gonzalez-Amaro, R., Cortes, J.R., Sanchez-Madrid, F. & Martin, P. Is CD69 an effective brake to control inflammatory diseases? Trends Mol. Med. 19, 625–632 (2013).
Bronte, V. et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 7, 12150 (2016).
Terabe, M. & Berzofsky, J.A. The immunoregulatory role of type I and type II NKT cells in cancer and other diseases. Cancer Immunol. Immunother. 63, 199–213 (2014).
Scott, D.J. et al. Lack of inducible nitric oxide synthase promotes intestinal tumorigenesis in the Apc(Min/+) mouse. Gastroenterology 121, 889–899 (2001).
Edin, S. et al. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS ONE 7, e47045 (2012).
Biswas, S.K., Allavena, P. & Mantovani, A. Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Semin. Immunopathol. 35, 585–600 (2013).
Wang, L., Wang, Y., Song, Z., Chu, J. & Qu, X. Deficiency of interferon-gamma or its receptor promotes colorectal cancer development. J. Interferon Cytokine Res. 35, 273–280 (2015).
Akeus, P. et al. Treg-cell depletion promotes chemokine production and accumulation of CXCR3(+) conventional T cells in intestinal tumors. Eur. J. Immunol. 45, 1654–1666 (2015).
Bjordahl, R.L., Gapin, L., Marrack, P. & Refaeli, Y. iNKT cells suppress the CD8+ T cell response to a murine Burkitt’s-like B cell lymphoma. PLoS ONE 7, e42635 (2012).
Renukaradhya, G.J., Sriram, V., Du, W., Gervay-Hague, J., Van Kaer, L. & Brutkiewicz, R.R. Inhibition of antitumor immunity by invariant natural killer T cells in a T-cell lymphoma model in vivo. Int. J. Cancer 118, 3045–3053 (2006).
Terabe, M. et al. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat. Immunol. 1, 515–520 (2000).
Terabe, M. et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J. Exp. Med. 198, 1741–1752 (2003).
Naito, Y. et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 58, 3491–3494 (1998).
Tosolini, M. et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 71, 1263–1271 (2011).
Bindea, G. et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 39, 782–795 (2013).
Schiering, C. et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 513, 564–568 (2014).
Vasanthakumar, A. et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat. Immunol. 16, 276–285 (2015).
Kolodin, D. et al. Antigen- and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell Metab. 21, 543–557 (2015).
Mertz, K.D. et al. The IL-33/ST2 pathway contributes to intestinal tumorigenesis in humans and mice. Oncoimmunology 5, e1062966 (2016).
Sag, D., Krause, P., Hedrick, C.C., Kronenberg, M. & Wingender, G. IL-10-producing NKT10 cells are a distinct regulatory invariant NKT cell subset. J. Clin. Invest. 124, 3725–3740 (2014).
Lynch, L. et al. Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of Treg cells and macrophages in adipose tissue. Nat. Immunol. 16, 85–95 (2015).
Vieth, J.A., Das, J., Ranaivoson, F.M., Comoletti, D., Denzin, L.K. & Sant'Angelo, D.B. TCRalpha-TCRbeta pairing controls recognition of CD1d and directs the development of adipose NKT cells. Nat. Immunol. 18, 36–44 (2017).
Fujii, S. et al. NKT cells as an ideal anti-tumor immunotherapeutic. Front. Immunol. 4, 409 (2013).
Acknowledgements
We thank Marianne Quiding-Järbrink for providing the ApcMin/+ mice, and Marianne Quiding-Järbrink and Fredrik Ivars for constructive comments to the manuscript. We thank Samuel Alsén for assistance with cell sorting. The NIH Tetramer Facility is gratefully acknowledged for provision of PBS57-loaded CD1d tetramers. This work was supported by grants from the Swedish Cancer Foundation and the Swedish Research Council, and support from the Strategic Research Center for Mucosal Immunobiology and Vaccines (MIVAC) to S.C., and grants from Assar Gabrielsson Foundation, Adlerbertska Research Foundation, and Wilhelm and Martin Lundgren Science Foundation to Y.W. and S.A.P. was supported by NIH RO1 Grant AI45889. Y.W. was financed by a PhD project grant and S.C. was financed from the Sahlgrenska Academy, University of Gothenburg.
Author contributions
Y.W. planned and performed experiments, analyzed data, and prepared manuscript figures and text. S.S. planned and performed experiments and reviewed the manuscript. L.L. performed experiments. S.P. provided reagents, designed experiments, and reviewed the paper. S.C. initiated and designed the study, analyzed data, and wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, Y., Sedimbi, S., Löfbom, L. et al. Unique invariant natural killer T cells promote intestinal polyps by suppressing TH1 immunity and promoting regulatory T cells. Mucosal Immunol 11, 131–143 (2018). https://doi.org/10.1038/mi.2017.34
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2017.34
This article is cited by
-
Macrophage barrier in the tumor microenvironment and potential clinical applications
Cell Communication and Signaling (2024)
-
Role of NKT cells in cancer immunotherapy—from bench to bed
Medical Oncology (2022)
-
MAP3K2 augments Th1 cell differentiation via IL-18 to promote T cell-mediated colitis
Science China Life Sciences (2021)
-
YY1lo NKT cells are dedicated IL-10 producers
Scientific Reports (2020)
-
Regulatory T cells specifically suppress conventional CD8αβ T cells in intestinal tumors of APCMin/+ mice
Cancer Immunology, Immunotherapy (2020)