Abstract
The gut CD4+ T cells, particularly the T helper type 17 (Th17) subset, are not completely restored in most HIV-1-infected individuals despite combined antiretroviral therapy, when initiated at the chronic phase of infection. We show here that the CCR6–CCL20 chemotactic axis is altered, with reduced CCL20 production by small intestine epithelial cells in treated HIV-1-infected individuals. This leads to impaired CCR6+CD4+ T-cell homing, particularly Th17 cells, to the small intestine mucosa. In contrast, the frequency of gut FoxP3+ T regulatory (Treg) cells, specifically the CCR6− subset, was increased. The resulting imbalance in the Th17/CCR6− Treg ratio and the associated shift from interleukin (IL)-17 to IL-10 and transforming growth factor-β (TGF-β) blunts CCL20 production by enterocytes, perpetuating a negative feedback for the recruitment of CCR6+CD4+ T cells to the small intestine in treated HIV-1-infected individuals.
Similar content being viewed by others
INTRODUCTION
The intestinal mucosa forms a vast barrier between the environment and the host. It comprises several lines of defense. First, mucus production, antimicrobial peptides, and immunoglobulins A minimize direct contacts between the intestinal pathogens and the epithelial cells. Second, the maintenance of a healthy epithelial barrier prevents penetration of pathogens. Lastly, innate and adaptive responses can be mounted against pathogens that have penetrated the intestinal tissue through breaches in the epithelial barrier.1
Lymphocytes are found in organized immune inductive sites (Peyer’s patches and mesenteric lymph nodes) in the gut and in diffuse effector sites along the intestinal epithelium and lamina propria. The gut CD4+ T cells are profoundly depleted during the course of HIV-1 infection.2, 3, 4, 5, 6 The T helper type 17 (Th17) cells, a T-cell subset that has a major role in antibacterial and antifungal mucosal defenses, are particularly concerned.7, 8 The great susceptibility of gut CD4+ T cells to HIV-1 is due to the activated status of memory CD4+ T cells in the gut mucosa, the abundance of cells expressing the CCR5 coreceptor for HIV-1 entry, and the affinity of the virus envelope glycoprotein Gp120 for α4β7, an heterodimeric integrin expressed by most gut T cells.9, 10, 11, 12 The resulting depletion of CD4+ T cells in the gut, particularly the Th17 cell subset, is a key factor in the pathophysiology of HIV-1 infection, as it is in pathogenic simian immunodeficiency virus (SIV) infection in Asian macaques.7, 8, 13
The loss of Th17 cells in the gut mucosa has been linked to microbial translocation from the gut lumen to the bloodstream, resulting in systemic inflammation and disease progression.14, 15, 16, 17 In contrast, gut Th17 cells are selectively preserved in nonpathogenic SIV infection of natural hosts like the African green monkeys and sooty mangabeys.8, 18 This could contribute to the absence of microbial translocation and systemic inflammation in these species, highlighting the importance of preserving or restoring gut Th17 cells of HIV-1-infected individuals.
The initiation of combined antiretroviral therapy (cART) early in HIV-1 primary infection could preserve gut T-cell homeostasis and the integrity of the epithelial barrier.19, 20 However, the restoration of CD4+ T cells appears to be delayed in most subjects who are started on cART at the chronic phase, and it is less complete in the lamina propria of the intestinal mucosa than in the peripheral blood and organized lymphoid tissues.4, 21 The gut mucosa remains particularly depleted in Th17 cells, despite sustained effective cART.8, 16, 20
We previously showed that a lack of CD4+ T cell recruitment to the gut could be involved in the incomplete restoration of CD4+ T cells in the intestinal mucosa.22 The main chemotactic axes involved in the homing of T lymphocytes to the small intestine are the α4β7–mucosal addressin cell adhesion molecule-1 (MAdCAM-1) and CCR9–CCL25 axes.23, 24, 25 The priming of lymphocytes at inductive sites of the gut-associated lymphoid tissue by CD103+ dendritic cells in the presence of retinoic acid, a vitamin A metabolite, induces high-level expression of α4β7 and CCR9.26, 27 The imprinted T cells expressing CCR9 and α4β7 are then prone to migrate from the blood to the effector sites of the small intestine mucosa. The α4β7 integrin binds MAdCAM-1 that is expressed on high endothelial venules of Peyer’s patches and mesenteric lymph nodes and on postcapillary venules of the gut lamina propria in both the small intestine and the colon.23 The chemokine CCL25 seems to be produced constitutively by epithelial cells of the small intestine.24, 25 Immune cells expressing CCR9 and α4β7 are thus prone to migrate to the small intestine specifically, suggesting that this segment of the gut has a specific immune function.
The CCR6–CCL20 axis also plays a critical role in the homing of immune cells to the gut, particularly that of dendritic cells to Peyer’s patches, and also Th17 and T regulatory (Treg) cell homing to the intestine lamina propria.28, 29, 30, 31, 32, 33 CCL20 is produced by epithelial cells of both the small intestine and the colon. It can also be induced by inflammatory stimuli, particularly cytokines like interleukin (IL)-1β and tumor necrosis factor-α (TNF-α), and some pathogen-associated molecular patterns through Toll-like receptor (TLR) signaling.34, 35 Segmented filamentous bacteria can notably induce ileal enterocytes to produce CCL20, and hence the homing of Th17 cells to the gut.36
The CCR6–CCL20 axis thus appears to play an important role in regulating the immune homeostasis of the gut mucosa. Th17 cells are markedly and selectively depleted in the intestinal mucosa during HIV-1 or pathogenic SIV infections, leading to an unbalanced Th17/Treg cell ratio that is correlated with systemic inflammation.18, 20 The Th17/Treg cell ratio in the gut mucosa remains lower in most HIV-1-infected than in uninfected subjects, even after sustained control of HIV-1 replication under cART, and it is correlated with the restoration of CD4+ T cells in the gut mucosa.20
We previously described an alteration in the CCR9–CCL25 chemotactic axis that contributes to the poor restoration of CD4+ T cell in the small intestine mucosa of treated HIV-1-infected subjects who initiated cART at the chronic phase of infection. The lack of recruitment of CD4+ T cells along the CCR9–CCL25 axis appears to be linked to the reduced production of CCL25 by small intestine epithelial cells.22
We have now focused on the homing of CCR6+CD4+ T cells to the small intestine mucosa, particularly the Th17, Th1Th17, and Treg cell subsets. Persistent microbial translocation and inflammatory stimuli in the gut mucosa of treated HIV-infected individuals should theoretically induce CCL20 production and the homing of CCR6+ T cells like Th17 cells to the gut, but previous reports have shown that gut Th17 cells are not restored in this setting.8, 19, 20 We therefore investigated the functional status of the CCR6–CCL20 axis in HIV-1-infected individuals on sustained effective cART and how it interacts with the α4β7–MAdCAM-1 and CCR9–CCL25 axes.
RESULTS
Reduced CCL20 production by enterocytes impairs the homing of CCR6+CD4+ T cells to the small intestine in treated HIV-1-infected individuals
We previously showed that CD4+ T cells remain depleted, both in percentage and absolute number, in the small intestine mucosa of most HIV-1-infected individuals despite prolonged effective cART.22 This contrasts with the adequate restoration of the blood CD4+ T count in these subjects (median 668 cells per μl; interquartile range (IQR), 451–849) who had been treated for a median of 5 years (IQR, 4–5.5). Changes in CD4+ T cells homing to the gut could contribute to the observed impaired mucosal immune reconstitution. We focused on the CCR6–CCL20 chemotactic axis as it governs Th17 cells homing.30, 31 The frequencies of CCR6+ in CD4+ T cells in the peripheral blood of treated HIV-1-infected individuals and uninfected controls were similar, as analyzed by flow cytometry (50% vs. 52%, P=0.29; Figure 1a), but the frequency in the small intestine mucosa of treated HIV-1-infected individuals was significantly reduced (46.8% vs. 60.4%, P=0.01; Figure 1a). There was no statistically significant correlation between CD4+ T-cell counts and the frequency of CCR6+CD4+ T cells in treated HIV-1-infected subjects, neither in the blood (ρ=0.31, P=0.19) nor in the gut mucosa (ρ=−0.41, P=0.21; data not shown).
Reduced frequency of CCR6+CD4+ T cells and altered expression of CCL20 in the small intestine mucosa of treated HIV-1-infected individuals. (a) Frequencies of CCR6+ cells in CD4+ T cells in the peripheral blood (PB) and small intestine mucosa of treated HIV-1-infected individuals (PB, n=20; gut, n=11) and uninfected individuals (PB, n=10; gut, n=11). Percentages of cells were determined by flow cytometry. (b) Correlation between the frequencies of CCR6+CD4+ T cells in the peripheral blood and small intestine mucosa in treated HIV-1-infected individuals (n=11). Each symbol represents one individual. (c) CCL20 mRNA expression in the small intestine epithelial cells of treated HIV-1-infected individuals (n=17) and uninfected individuals (n=14). CCL20 mRNA was quantified by real-time reverse transcriptase-PCR (RT-PCR) and normalized to GAPDH. AU, arbitrary unit. (d, e) CCL20 chemokine production in the small intestine mucosa. (d) CCL20 chemokine in the small intestine mucosa of treated HIV-1-infected individuals (n=9) and uninfected individuals (n=5) was quantified per unit surface area using NIS-element (Nikon). (e) Representative treated HIV-1-infected and uninfected individuals are shown. CCL20 (magenta) was stained by immunohistochemistry. Cell nuclei are counterstained (blue) (LSM 710 Confocal microscope, original magnification × 200).
The frequencies of CCR6+ in CD4+ T cells in the peripheral blood and small intestine mucosa were inversely correlated in treated HIV-1-infected individuals (ρ=−0.65, P=0.03; Figure 1b), suggesting cell trafficking between the two compartments. We determined the production of the chemokine CCL20, the ligand of CCR6, in the small intestine mucosa by measuring CCL20 mRNA in the enterocytes by real-time reverse transcriptase-PCR (RT-PCR). Treated HIV-1-infected individuals had 6 times less CCL20 mRNA in the enterocytes than uninfected controls (P=0.0009; Figure 1c). We also estimated CCL20 production in duodenojejunal tissue by immunohistochemistry. CCL20 staining was significantly reduced in the small intestine mucosa of treated HIV-1-infected individuals (quantification of CCL20 per unit surface area, P=0.004; Figure 1d), in agreement with the mRNA data, with stain almost exclusively of epithelial cells (Figure 1e). These results suggest that the reduced production of CCL20 by the enterocytes is involved in the altered homing of CCR6+CD4+ T cells to the small intestine mucosa of treated HIV-1-infected individuals.
The frequency of CCR6+CD4+ T cells in the small intestine mucosa was not correlated with HIV-1 DNA (ρ=0.02, P=0.96; Figure 2a) or residual RNA loads in gut tissue (ρ=−0.26, P=0.47; Figure 2b), arguing against a major role of HIV-1 residual replication in the reduced frequency of CCD6+CD4+ T cells in the gut mucosa of treated HIV-1-infected individuals.
Lack of correlation between HIV-1 DNA or RNA and the frequency of CCR6+CD4+ T cells in the small intestine mucosa of treated HIV-1-infected individuals. (a) Correlation between HIV-1 DNA in gut CD4+ T cells (copies per 106 cells) or (b) HIV-1 RNA in gut tissue (copies per mg tissue) and the frequency of CCR6+CD4+ T cells in the small intestine mucosa of treated HIV-1-infected individuals (n=11 for HIV-1 DNA; n=10 for HIV-1 RNA). Each symbol represents one individual. HIV-1 DNA/RNA was quantified by real-time PCR/reverse transcriptase-PCR (RT-PCR) with primer pairs and Taqman probes specific for LTR-gag on a Light-Cycler (Roche). Percentages of CCR6+CD4+ T cells were determined by flow cytometry.
The lack of Th17 cells contrasts with an increased frequency of CCR6− Tregs in the small intestine mucosa of treated HIV-1-infected individuals
We looked in more detail into CCR6+CD4+ T cells trafficking to the gut of treated HIV-1-infected individuals by analyzing the main CCR6+ T-cell subsets involved in mucosal immunity. We used flow cytometry to determine the frequencies of Th17, Th1Th17, and Treg cells in the peripheral blood and small intestine mucosa, based on their CD3+CD4+CCR4+CXCR3−CCR6+CD161+, CD3+CD4+CCR4−CXCR3+CCR6+CD161+, and CD3+CD4+CD25highCD127lowFoxP3+ phenotypes, respectively (representative experiments are shown in Figure 3a,b for lymphocytes from the peripheral blood, and in Supplementary Figure S1a,b online for lymphocytes from the small intestine mucosa).30, 31, 37, 38, 39
Reduced frequencies of T helper type 17 (Th17) and Th1Th17 cells but increased frequency of CCR6− T regulatory (Treg) cells in the small intestine mucosa of treated HIV-1-infected individuals. (a, b) Flow cytometry gating strategy for Th17 cells (CD3+CD4+CCR4+CXCR3−CCR6+CD161+) and Th1Th17 cells (CD3+CD4+CCR4−CXCR3+CCR6+CD161+) (a), and for Treg cells (CD3+CD4+CD25highCD127lowFoxP3+) (b). A representative experiment is shown for lymphocytes from the peripheral blood. The frequencies of Th17, Th1Th17, and Treg cells shown in Figure 2c–h were calculated as the percentage of CCR4+CXCR3−CCR6+CD161+ cells among CD3+CD4+ T cells; the percentage of CCR4−CXCR3+CCR6+CD161+ cells among CD3+CD4+ T cells, and the percentage of CCR25highCD127lowFoxP3+ cells among CD3+CD4+ T cells, respectively. (c, d) Frequencies of Th17 cells (c) and Th1Th17 cells (d) in CD4+ T cells in the peripheral blood (PB) and small intestine mucosa of treated HIV-1-infected individuals (PB, n=20; gut, n=11) and uninfected individuals (PB, n=10; gut, n=11). (e) Frequencies of Treg cells in CD4+ T cells in the PB and small intestine mucosa of treated HIV-1-infected individuals (PB, n=20; gut, n=11) and uninfected individuals (PB, n=10; gut, n=11). (f) Frequencies of CCR6+ cells in Treg cells in the small intestine mucosa of treated HIV-1-infected individuals (n=11) and uninfected individuals (n=11). (g) Frequencies of CCR6− Treg cells in CD4+ T cells in the small intestine mucosa of treated HIV-1-infected (n=11) and uninfected (n=11) individuals.
The frequencies of Th17, Th1Th17, and Treg in CD4+ T cells in the peripheral blood of treated HIV-1-infected individuals and uninfected controls did not differ significantly (1.2% vs. 2% for Th17 cells, P=0.13, Figure 3c; 1.3% vs. 2% for Th1Th17 cells, P=0.35, Figure 3d; and 15.8% vs. 13.3% for Treg cells, P=0.17, Figure 3e). However, Th17 cells were significantly less frequent in the CD4+ T cells of the small intestine mucosa of treated HIV-1-infected individuals than in controls (4.3% vs. 7.7%, P=0.02; Figure 3c), as was the case for Th1Th17 cells (4.2% vs. 6.8%, P=0.01; Figure 3d). In contrast, Treg cells were more frequent in the CD4+ T cells in the small intestine mucosa of treated HIV-1-infected individuals than in uninfected controls (28.2% vs. 18.1%, P=0.008; Figure 3e).
We then analyzed the CCR6+ and CCR6− subsets of Treg cells. Although the frequency of Treg cells as a whole in the small intestine mucosa of treated HIV-1-infected individuals was increased, there were fewer CCR6+ among the Treg cells than in uninfected controls (49.8% vs. 69.8%, P=0.07; Figure 3f). Thus, the increased frequency of Treg cells in CD4+ T cells in the small intestine mucosa of treated HIV-1-infected individuals was because of their CCR6− subset (13.5% vs. 6.43%, P=0.001; Figure 3g), whose homing to the gut does not depend on the CCR6–CCL20 axis.
The gut Th17/CCR6− Treg ratio is correlated with the homing of CCR6+CD4+ T cells to the small intestine in treated HIV-1-infected individuals
The CCR6+ subset of Treg cells depends on the CCR6–CCL20 axis, as Th17 cells, to migrate to the gut. The CCR6+ and CCR6− subsets of Treg cells should be considered separately, as they change differently in the small intestine mucosa of treated HIV-1-infected individuals. We therefore considered only the Th17/CCR6− Treg and Th1Th17/CCR6− Treg ratios in further analyses. The Th17/CCR6− Treg ratio was markedly lower in treated HIV-1-infected individuals than in uninfected controls (0.32 vs. 1.45, P=0.001; Figure 4a), as was the Th1Th17/CCR6− Treg ratio (0.33 vs. 1.40, P=0.002; Figure 4b).
Correlation between the Th17/CCR6− T regulatory (Treg) ratio and the homing of CCR6+CD4+ T cells to the small intestine mucosa. (a, b) Th17/CCR6− Treg ratio (a) and Th1Th17/CCR6− Treg ratio (b) in the small intestine mucosa of treated HIV-1-infected (n=11) and uninfected (n=11) individuals. (c–e) Negative correlation between the frequency of CCR6− Treg cells and the frequencies of CCR6+CD4+ T cells (c), T helper type 17 (Th17) cells (d), and Th1Th17 cells (e) in the small intestine mucosa (11 treated HIV-1-infected and 11 uninfected individuals). (f, g) Correlation between the Th17/CCR6− Treg ratio (f) and Th1Th17/CCR6− Treg ratio (g) and the frequency of CCR6+CD4+ T cells in the small intestine mucosa (11 treated HIV-1-infected and 11 uninfected individuals). HIV-1−, white symbol; HIV-1+, gray symbol.
The frequency of CCR6− Treg cells in the small intestine mucosa was negatively correlated with the frequency of CCR6+CD4+ T cells (ρ=−0.87, P<0.0001; Figure 4c), Th17 cells (ρ=−0.64, P=0.001; Figure 4d), and Th1Th17 cells (ρ=−0.56, P=0.007; Figure 4e). These data suggest that as more CCR6− Treg cells are located in the small intestine lamina propria, the fewer CCR6+CD4+ T cells, notably Th17 and Th1Th17, migrate to it.
Both the Th17/CCR6− Treg and Th1Th17/CCR6− Treg ratios and the frequency of CCR6+CD4+ T cells in the small intestine mucosa were strongly correlated (ρ=0.86 for the Th17/CCR6− Treg ratio, P≤0.0001, Figure 4f; ρ=0.92 for the Th1Th17/CCR6− Treg ratio, P≤0.0001, Figure 4g). The shift from Th17 to CCR6− Treg cells in the small intestine mucosa of treated HIV-1-infected individuals could be a consequence of the alteration of the CCR6–CCL20 chemotactic axis, but it could also be a cause of it if this imbalance negatively affects CCL20 production.
The α4β7–MAdCAM-1 axis, but not the CCR9–CCL25 one, partly offsets the defective homing of CCR6+CD4+ T cells along the CCR6–CCL20 axis in treated HIV-1-infected individuals
We then examined whether Th17, Th1Th17, and Treg cells could migrate from the blood to the small intestine mucosa along the CCR9–CCL25 and α4β7–MAdCAM-1 chemotactic axes in addition to the CCR6–CCL20 axis. We analyzed the expression of CCR9 and α4β7 on Th17, Th1Th17, and Treg cell subsets by flow cytometry. Most of the gut Th1Th17 cells expressed CCR9, whereas only a few Th17 cells did so (61.3% vs. 5.9% in treated HIV-1-infected individuals; P=0.003; Figure 5a). More of the gut CCR6+ Treg cells expressed CCR9 than their CCR6− counterparts (34.5% vs. 22.3% in treated HIV-1-infected individuals, P=0.003; Figure 5a). The β7 integrin was expressed on most of the gut Th17 (91.3%), Th1Th17 (84.6%), and Treg cells, both CCR6− (74.5%) and CCR6+ cells (81%) in treated HIV-1-infected individuals (Figure 5b). Figure 5c shows the expression of CCR9 and β7 integrin on Th17, Th1Th17, and Treg cell subsets in the peripheral blood and small intestine mucosa of a representative treated HIV-1-infected individual. These results suggest that gut-tropic Th17 cells do not depend on the CCR9–CCL25 axis to migrate to the small intestine mucosa in addition to the CCR6–CCL20 and α4β7–MAdCAM-1 axes, although this chemotactic axis could be important for Th1Th17 cells.
Interplay between the CCR6-CCL20, CCR9-CCL25, and α4β7–MAdCAM-1 chemotactic axes for the homing of T helper type 17 (Th17), Th1Th17, and T regulatory (Treg) cells to the small intestine mucosa in treated HIV-1-infected individuals. (a, b) Frequencies of CCR9+ cells (a) and β7+ cells (b) in the Th17, Th1Th17, CCR6+ and CCR6− Treg cell subsets in the small intestine mucosa of treated HIV-1-infected individuals (n=11). Percentages of cells were determined by flow cytometry. (c) Flow cytometry analysis of the expression of CCR9 and β7 integrin on Th17, Th1Th17, CCR6+ and CCR6− Treg cells in the peripheral blood (PB) and small intestine mucosa of a representative treated HIV-1-infected individual. The β7 staining was separated into three categories (β7−, β7int, and β7high) for gut lymphocytes. (d) Correlation between mucosal addressin cell adhesion molecule-1 (MAdCAM-1) amounts and the frequency of CCR6+CCR9+β7high in CD4+ T cells in the small intestine mucosa of treated HIV-1-infected individuals (n=10). Each symbol represents one individual. (e) Frequencies of CCR6+ cells in CD8+ T cells in the small intestine mucosa of treated HIV-1-infected individuals (n=11) and uninfected individuals (n=11). Percentages of cells were determined by flow cytometry. (f) Correlation between MAdCAM-1 amounts and the frequency of CCR6+β7high in CD8+ T cells in the small intestine mucosa of treated HIV-1-infected individuals (n=10). Each symbol represents one individual. (g) Frequencies of CXCR3+ in CCR6+CD8+ cells in treated HIV-1-infected (n=11) and uninfected individuals (n=11). Percentages of cells were determined by flow cytometry. AU, arbitrary unit.
We then examined the function of these axes and how they could compensate for one another. We have previously shown that the CCR9–CCL25 axis is altered in treated HIV-1-infected individuals because of the reduced production of CCL25 by small intestine epithelial cells. This is correlated with fewer CCR9+CD4+ T cells in the small intestine mucosa that is mirrored by more abundant gut-tropic CCR9+CD4+ T cells in the peripheral blood.22 Our present results for CCR9+ cells in the Th17 and Th1Th17 and Treg cell subsets were similar (data not shown). We also showed using immunohistochemistry that the amount of MAdCAM-1 on the endothelial cells of the gut lamina propria of treated HIV-1-infected individuals was similar to that of uninfected controls.22 Thus, the α4β7–MAdCAM-1 axis could function in treated HIV-1-infected individuals. The amount of MAdCAM-1 was positively correlated with the frequency of CCR6+CCR9+β7high CD4+ T cells in the gut of treated HIV-1-infected individuals (ρ=0.69, P=0.03; Figure 5d). The amount of MAdCAM-1 and the gut frequencies of the CCR6+CCR9+β7high subsets of Th17, Th1Th17, and Treg cells also tended to be correlated (ρ=0.54, P=0.09 for Th17; ρ=0.60, P=0.05 for Th1Th17; and ρ=0.53, P=0.09 for Treg cells, data not shown). This suggests that the α4β7–MAdCAM-1 axis is functioning and drives some CCR6+CCR9+β7+ cells to the small intestine mucosa as an alternative to the defective CCR6–CCL20 and CCR9–CCL25 axes. However, it cannot fully compensate for the defective homing of CCR6+CD4+ T cells along the CCR6–CCL20 axis as Th17 and Th1Th17 cells in the small intestinal mucosa of treated HIV-1-infected individuals remained markedly depleted despite effective cART.
In contrast to the observed depletion of CCR6+CD4+ T cells in the gut mucosa, the frequencies of CCR6+CD8+ T cells in HIV-1-infected individuals and uninfected controls were similar (52.6% vs. 58.8%, P=0.14; Figure 5e). Thus, the defect in the CCR6–CCL20 axis seems to affect the CD4+ and CD8+ T cell populations differently in HIV-1-infected individuals, as is the case for the CCR9–CCL25 axis.22 We previously showed that gut CD8+ T cells express higher levels of β7 integrin than do CD4+ T cells.22 The α4β7–MAdCAM-1 axis seems to be functional, as we found a strong positive correlation between the levels of MAdCAM-1 and the frequency of CCR6+β7highCD8+ T cells in the small intestine mucosa of treated HIV-1-infected individuals (ρ=0.80, P=0.006; Figure 5f). Moreover, the frequency of CXCR3+CCR6+CD8+ T cells in the small intestine mucosa of treated HIV-1-infected individuals was greater than in uninfected controls (35.3% vs. 23.6%, P=0.02; Figure 5g). These results suggest that the trafficking of CD8+ T cells to the gut depends more on α4β7 and/or CXCR3-driven chemotaxis than on CCR6 and/or CCR9 in this setting.
CCR6− and CCR6+ Treg cells display different chemokine receptors and cytokine production profiles
The frequency of CCR6− Treg cells in the small intestine mucosa of treated HIV-1-infected individuals was increased, whereas that of CCR6+ Treg and Th17/Th1Th17 cells was reduced. We thus examined the phenotype of CCR6− and CCR6+ Treg cells. In addition to the α4β7 integrin and CCR9 chemokine receptor, more of the CCR6+ Treg cells expressed CCR4, CCR5, and CCR10 than their CCR6− counterparts in treated HIV-1 infected individuals (50.8% vs. 28.8% for CCR4, P=0.04; 28.8% vs. 17.5% for CCR5, P=0.08; 14% vs. 4.9% for CCR10, P=0.04; Figure 6a). Both the CCR6+ and CCR6− Treg cell subsets expressed CXCR4 at similar intensities (data not shown). In contrast, more of the CCR6− Treg cells expressed CXCR3 than their CCR6+ counterparts (37.7% vs. 27.8%, P=0.04; Figure 6a), and the frequency of CXCR3+CCR6− Treg cells in CD4+ T cells was higher in treated HIV-1-infected individuals than in uninfected controls (37.7% vs. 16.9%, P=0.02; Figure 6b). Thus, CXCR3-driven chemotaxis could contribute to the homing of CCR6− Treg cells to the small intestine mucosa.
Chemokine receptors and cytokine production profiles of CCR6− and CCR6+ T regulatory (Treg) cells. (a) Frequencies of CCR4+, CCR5+, CCR10+, and CXCR3+ cells in CCR6− and CCR6+ Treg cell subsets in the peripheral blood (PB) of treated HIV-1-infected individuals (n=5). Percentages of cells were determined by flow cytometry. (b) Frequencies of CXCR3+ in CCR6− Treg cells in treated HIV-1-infected (n=5) and uninfected individuals (n=5). (c) Production of interleukin-10 (IL-10) and transforming growth factor-β1 (TGF-β1) by CCR6− and CCR6+ Treg cell subsets. (d) Production of IL-17 by T helper type 17 (Th17) cells and CCR6− and CCR6+ Treg cell subsets. Cells were sorted from PB by flow cytometry, cultured, and stimulated ex vivo. Cytokines secreted in the culture medium were quantified by enzyme-linked immunosorbent assay (ELISA). The mean and s.d. is shown (n=4 independent experiments performed with different donors). (e) Phenotype of IL-17- and IL-10-producing Treg cells in the PB and gut mucosa. The frequencies of CCR6+ (white bars) and CCR6− (black bars) cells among IL-17- and IL-10-producing Treg cells are shown. The cells were stimulated by phorbol 12-myristate 13-acetate (PMA)/ionomycin before IL-17 and IL-10 staining and flow cytometry analysis. The mean is shown (n=6 individuals for PB analysis, and n=4 individuals for gut mucosa analysis). *P<0.05; **P<0.01.
CCR6− and CCR6+ Treg cells also differed regarding their cytokine production profiles. We sorted CCR6− and CCR6+ Treg cells and Th17 cells from peripheral blood mononuclear cells by flow cytometry. The sorted cells were cultured ex vivo and characterized regarding their cytokine production profiles by enzyme-linked immunosorbent assay. CCR6− Treg cells produced more IL-10 than CCR6+ Treg cells (P=0.02; Figure 6c), whereas both Treg cell subsets produced similar levels of transforming growth factor-β (TGF-β, P=0.12; Figure 6c). CCR6+ Treg cells mainly differ from their CCR6− counterparts regarding IL-17 production (P=0.02; Figure 6d). CCR6+ Tregs thus produced significant amounts of IL-17, although at a lesser extent than Th17 cells (approximately fourfold less, P=0.03). These data were confirmed in vivo in peripheral blood and gut mucosa by flow cytometry. The vast majority of IL-17-producing Treg cells were in the CCR6+ fraction of Treg cells, both for peripheral blood and gut Treg cells, as analyzed by flow cytometry (P=0.005; Figure 6e). In contrast, most IL-10-producing Treg cells were in the CCR6− fraction of Treg cells (P=0.018; Figure 6e). Thus, IL-17-producing cells, mainly Th17 cells, and also the CCR6+ subset of Treg cells that depend on the CCR6–CCL20 chemotactic axis, were reduced in the small intestine mucosa of treated HIV-1-infected individuals, whereas CCR6− Tregs that display different chemokine receptors and cytokine production profiles were increased.
The imbalance of the Th17/CCR6− Treg ratio in favor of the CCR6− Treg cells and the associated shift from IL-17 to IL-10 and TGF-β blunts CCL20 production by enterocytes
The shift from Th17 to CCR6− Treg cells could be both a consequence of the alteration of the CCR6–CCL20 chemotactic axis and a cause of it. We therefore examined the impact of the cytokines produced by the Th17 and Treg cell subsets on CCL20 production by small intestine epithelial cells. We developed an ex vivo model of human primary small intestine epithelial cells cultured as monolayers of differentiated enterocytes on transwell inserts (Supplementary Figure S2) in order to further investigate the factors modulating CCL20 production by enterocytes. We measured CCL20 production by real-time RT-PCR (CCL20 mRNA) and enzyme-linked immunosorbent assay (CCL20 protein). CCL20 production can be induced by several inflammatory stimuli. We used IL-1β and TNF-α as positive controls (data not shown). Activating TLRs 1 to 6 triggered CCL20 production by enterocytes as did activating NOD2-like receptor, although the response was smaller (Supplementary Figure S3). Thus, microbial sensing induced enterocytes to produce CCL20. This contrasts with the reduced CCL20 production in treated HIV-1-infected individuals. We then determined whether the cytokines produced by the Th17 and Treg cell subsets in the gut lamina propria could modulate CCL20 production by enterocytes.
Stimulation with IL-17A increased CCL20 production by the enterocytes in our ex vivo model (Figure 7a). Thus IL-17-producing cells, mainly Th17 cells, and also the CCR6+ subset of Treg cells, could create a positive feedback loop by secreting IL-17 that then induces CCL20 production, hence promoting the recruitment of CCR6+ cells to the small intestine mucosa. A lack of responsiveness of the enterocytes to IL-17 through its receptor (IL-17R) could contribute to the reduced CCL20 production in treated HIV-1-infected individuals. We quantified IL-17RA mRNA in the enterocytes from small intestine biopsies by real-time RT-PCR. The median amount of IL-17RA mRNA in treated HIV-1-infected individuals was lower than that in uninfected controls (P=0.013, Supplementary Figure S4a). We also quantified IL-8 (CXCL8) mRNA, as production of this chemokine by the enterocytes can also be induced by IL-17A via IL-17RA-induced NF-κB signaling, as it is the case for CCL20.34, 40, 41 We found that IL-8 mRNA was also less abundant in treated HIV-1-infected individuals than in uninfected controls (P=0.011, Supplementary Figure S4b), and that the levels of IL-8 mRNA were strongly correlated with those of IL-17RA mRNA (ρ=0.80, P=0.0004, Supplementary Figure S4c), suggesting a link between the reduced interleukin 17A receptor (IL-17RA) expression by the enterocytes and the low IL-8 production. However, the levels of CCL20 mRNA and those of IL-17RA mRNA were not statistically significantly correlated (ρ=0.45, P=0.10, Supplementary Figure S4d), suggesting that the reduced CCL20 production in treated HIV-1-infected individuals depends not only on insufficient IL-17 in the microenvironment and/or on a lack of responsiveness of the enterocytes through IL-17R, but also on additional regulatory factors that are involved.
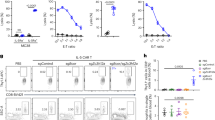
Impact of the shift from T helper type 17 (Th17) to CCR6− T regulatory (Treg) cells on CCL20 production by small intestine epithelial cells cultured ex vivo. (a) CCL20 mRNA expression in primary small intestine epithelial cells treated for 15 h with 0.5, 5, or 50 ng ml−1 interleukin (IL)-17A, IL-10, or transforming growth factor-β1 (TGF-β1). Primary small intestine epithelial cells were cultured as monolayers of differentiated enterocytes on transwell inserts. Presented data were obtained from three independent experiments performed with different donors. CCL20 mRNA expression in the enterocytes was quantified by real-time reverse transcriptase-PCR (RT-PCR) and normalized to GAPDH. (b) CCL20 expression by primary small intestine epithelial cells after their coculture with various proportions of Th17 and CCR6− Treg cells. CCL20 mRNA in the enterocytes was quantified by real-time RT-PCR and normalized to GAPDH (black bars); CCL20 secreted in the culture medium was quantified by enzyme-linked immunosorbent assay (ELISA) (white bars). Results are presented as fold change of CCL20 when the Th17/CCR6− Treg ratio shifts from its physiological value in uninfected individuals in favor of the Th17 cells (left) or in favor of the CCR6− Treg cells (right), as it is the case in HIV-1-infected individuals. The Th17/CCR6− Treg ratios measured in the small intestine mucosa of uninfected (physiological ratio of 3/2) and treated HIV-1-infected individuals (pathological ratio of 1/3) are indicated by open and filled arrows, respectively. AU, arbitrary unit. (c) Positive correlation between IL-17 and CCL20 protein levels. (d, e) Negative correlations between IL-10 (d) or TGF-β1 (e), and CCL20 protein levels. The protein levels were measured by ELISA in coculture experiments (n=8).
Regarding the potential role of the cytokines produced by Treg cells on CCL20 production by the enterocytes, we found that both IL-10 and TGF-β1 decreased it in our ex vivo model (Figure 7a). Thus, CCR6− Treg cells could negatively regulate the CCR6–CCL20 chemotactic axis by secreting IL-10 and TGF-β1 but not IL-17.
We cocultured ex vivo primary small intestine epithelial cells with various proportions of Th17 and CCR6− Treg cells sorted by flow cytometry and placed in the bottom chamber of the transwells to mimic lamina propria–epithelium interactions. The production of both CCL20 mRNA and CCL20 protein (Figure 7b) were blunted when the Th17/CCR6− Treg ratio shifted in favor of the CCR6− Treg cells. The production of CCL20 was reduced by fourfold (CCL20 protein) to sixfold (CCL20 mRNA) when the Th17/CCR6− Treg ratio shifted from a physiological value (1.6 in our uninfected controls) to what was observed in treated HIV-1-infected individuals (0.35), in agreement with our data in vivo. The levels of secreted CCL20 protein were positively correlated with the concentration of IL-17 (ρ=0.93, P=0.0007; Figure 7c), whereas they were negatively correlated with those of IL-10 (ρ=−0.83, P=0.01; Figure 7d) and TGF-β1 (ρ=−0.71, P=0.04; Figure 7e). Therefore, these results suggest that a low Th17/CCR6− Treg ratio in the gut, and the associated shift from IL-17 to IL-10 and TGF-β, prevents the normal production of CCL20 by enterocytes that blunts the homing of CCR6+CD4+ T cells, in particular Th17 cells, to the small intestine mucosa along the CCR6–CCL20 axis, perpetuating a vicious circle in treated HIV-1-infected individuals despite effective cART.
DISCUSSION
The CD4+ T cells in the gut mucosa lamina propria are not completely restored in most treated HIV-1-infected individuals who began cART at the chronic phase, in contrast to those of the peripheral blood and organized lymphoid tissues.4, 21 This impaired mucosal immune barrier is associated with persistent low-level microbial translocation from the gut microbiota into the bloodstream, fueling chronic systemic inflammation in HIV-1-infected individuals despite effective cART.14, 15, 16, 17 We previously described how the altered homing of CD4+ T cells to the small intestine mucosa along the CCR9–CCL25 chemotactic axis could contribute to this poor gut immune reconstitution.22 We have now investigated the functioning of the CCR6–CCL20 axis, as this chemotactic axis specifically governs the homing of Th17 cells to the gut.30, 31, 33 Our findings indicate that the homing of CCR6+CD4+ T cells to the small intestine mucosa is impaired in treated HIV-1-infected individuals, mainly because of reduced CCL20 production by enterocytes. This defect was associated with the impaired restoration of Th17 and Th1Th17 cells in the small intestine mucosa. In contrast, Treg cells were more frequent, but only their CCR6− subset. The resulting shift from IL-17 to IL-10 and TGF-β1 blunts CCL20 production by enterocytes, creating a negative feedback loop for the recruitment of CCR6+CD4+ T cells to the small intestine mucosa.
A loss of the balance between Th17 and Treg cells in the gut mucosa has been associated with disease progression in replicative pathogenic SIV infection in pigtailed macaques, whereas the Th17/Treg ratio remains unchanged in nonpathogenic SIV infection in African green monkeys.8, 18 A shift from Th17 to Treg cells has also been associated with persistent CD4+ T-cell depletion in the gut mucosa despite cART in HIV-1-infected individuals.20 Initiation of cART early during HIV-1 primary infection resulted in a better immune reconstitution of gut CD4+ T cells than when cART initiation was late, at the chronic phase of infection.20 However, the Th17 cell population in the gut mucosa was not fully restored even in those subjects who started cART early, except for those who had initiated cART during the Fiebig I/II stages of HIV-1 primary infection.19 This suggests that the homeostasis of the gut mucosa is disrupted early in HIV-1 infection, persists despite effective cART, and prevents the restoration of a normal gut Th17 cell population.
In the setting of replicative HIV/SIV infections, CCL20 could play a role in recruiting CCR6+ cells to inflamed tissues.18, 42, 43, 44 CCR6+CD4+ T cells could be preferentially targeted by SIV/HIV as they are highly permissive to infection.45, 46, 47 Under effective cART, residual HIV-1 replication in the gut could contribute to the poor restoration of mucosal CD4+ T cells, particularly of CCR6+CD4+ T cells. Although HIV-1 RNA was detected in the small intestine mucosa of all the individuals tested despite cART, the absolute HIV-1 RNA load in small intestine tissue was low, and we found no correlation between it and depletion of any of the CCR6+CD4+ T-cell subsets. Thus, our results do not suggest that residual HIV-1 replication plays a direct role in the poor restoration of CCR6+CD4+ T cells in the small intestine mucosa in this population of treated HIV-1-infected individuals with sustained undetectable plasma virus load for many years. Further arguing against a direct role of HIV-1 replication, we found that CCL20 and CCL25 production were increased by HIV-1 replication in an ex vivo model of small intestine histocultures infected by a R5-tropic virus (data not shown), in contrast to the reduced levels of CCL20 and CCL25 in the small intestine mucosa of treated HIV-1-infected individuals. Our results thus suggest that the reduced frequencies of CCR6+CD4+ T cells in the gut could best be explained by a lack of recruitment rather than by persistent HIV-1 replication.
Most studies on HIV-1-infected individuals have been performed on biopsies from the rectum or the distal colon. The CD4+ T lymphocyte populations in the small intestine and the colon/rectum could differ; for example, most Th17 cells seem to be located in the small intestine mucosa.33, 48 The chemotactic axes involved in T-cell homing to the small intestine and colon are also different; the CCR9–CCL25 axis is only involved in homing to the small intestine mucosa.24, 25 The conclusions drawn from studies on immune recovery in the small intestine and the colon could therefore differ significantly.
Differences could also arise from the definition of Th17 and Th1Th17 cells. We defined them based on their surface phenotypes and not on the production of IL-17 and interferon-γ (IFN-γ). The phorbol 12-myristate 13-acetate/ionomycin stimulation required for intracellular cytokine staining strongly downregulates some chemokine receptors, particularly CCR9, preventing the correct phenotyping of stimulated T lymphocytes defined by their cytokine production profile. We therefore defined Th17 cells as CD3+CD4+CCR4+CXCR3-CCR6+CD161+ cells, and Th1Th17 cells as CD3+CD4+CCR4-CXCR3+CCR6+CD161+ cells, that have clearly been associated with IL-17+ IFN-γ- and IL-17+ IFN-γ+ phenotypes, respectively, for peripheral blood lymphocytes.30, 31, 37, 38 Regarding Treg cells, several FoxP3+ Treg cell subsets have previously been described, with different cytokine production profiles.49, 50, 51, 52, 53 CCR6+ Treg cells can produce IL-17 while retaining FoxP3 expression. Both CCR6+ and CCR6− Treg subsets retain suppressive function.49, 50, 51, 52, 53 We found that IL-17-producing cells, i.e., Th17, Th1Th17, and CCR6+ Treg cells, were markedly depleted in the small intestine mucosa of treated HIV-1-infected individuals in contrast to the increased frequency of CCR6− Treg cells, leading to an imbalanced Th17/CCR6− Treg cell ratio.
When we examined the alternative chemotactic axes that could be used by CCR6− Treg cells to reach the small intestine mucosa, we found that most gut-tropic Treg cells, both the CCR6+ and CCR6− subsets, expressed α4β7 integrin, whereas there was significantly more CCR9 on CCR6+ Treg cells than on their CCR6− counterparts. Thus, the defective CCR6–CCL20 and CCR9–CCL25 axes could both contribute to the lack of recruitment of CCR6+ Treg cells to the small intestine mucosa. In contrast, the α4β7–MAdCAM-1 axis seems to be functional, as we found similar amounts of MAdCAM-1 on the endothelial cells of treated HIV-1-infected individuals and uninfected controls. We also found positive correlations between the amounts of MAdCAM-1 and the frequencies of CCR6+CCR9+β7high subsets of Th17, Th1Th17, and Treg cells, and also with the frequency of CCR6+β7high CD8+ T cells in the small intestine mucosa, suggesting that the α4β7–MAdCAM-1 axis partly compensates for the defective CCR6–CCL20 and CCR9–CCL25 axes. More of the CCR6+ Treg cells expressed CCR4, CCR5, and CCR10 than their CCR6− counterparts, suggesting that these chemotactic axes are not driving the preferential recruitment of CCR6− Treg cells to the small intestine mucosa in treated HIV-1-infected individuals. However, more of the CCR6− Treg cells expressed CXCR3 than their CCR6+ counterparts, and the frequency of CXCR3+CCR6− Treg cells in CD4+ T cells was higher in treated HIV-1-infected subjects than in uninfected controls. This chemotactic axis could thus contribute to the homing of CCR6− Treg cells to the small intestine mucosa, as it could be the case for the homing of CD8+ T cells. We have preliminary results showing that CXCR3+ Th1 lymphocytes are more frequent in the small intestine mucosa of treated HIV-1-infected individuals than in uninfected controls, also suggesting that CXCR3-driven chemotaxis is functioning for some cell subsets (data not shown). This warrants further studies to explore the roles of CXCL9, CXCL10, and CXCL11, the ligands of CXCR3, in this setting. In contrast, Th1Th17, which is CXCR3+, remained depleted in the small intestine mucosa of treated HIV-1-infected individuals. We found that most of Th1Th17 cells expressed CCR9, whereas only a few Th17 cells did so, suggesting that the CCR9–CCL25 chemotactic axis is essential for the gut homing of Th1Th17 cells. CXCR3- and α4β7-driven chemotaxis may not be sufficient to offset the defective CCR9–CCL25 and CCR6–CCL20 axes for Th1Th17 cells recruitment to the small intestine mucosa.
We assessed the impact of IL-17, IL-10, and TGF-β cytokines on CCL20 production using small intestine epithelial cells cultured ex vivo as monolayers of polarized enterocytes. The differentiation of the enterocytes was checked by their expression of cytokeratine (CK)-18 and CK-20, by the organization of tight junctions revealed by continuous ZO-1 staining, and the integrity of the epithelium was checked by measuring transepithelial electrical resistance. The relevance of our results is supported by the use of human primary epithelial cells from the small intestine rather than colonic cell lines, and by the cocultures of primary enterocytes on inserts and Th17 and Treg cells placed in the bottom chamber of the transwells to mimic lamina propria–epithelium interactions. We found that CCL20 production by enterocytes is driven by IL-17-producing cells, creating a positive feedback loop that drives the further recruitment of CCR6+ T cells to the gut mucosa. In contrast, CCR6− Treg cells blunted CCL20 production by enterocytes, disrupting the CCR6–CCL20 chemotactic axis. In treated HIV-1-infected individuals, the shift from CCR6+ IL-17-producing cells to CCR6− cells in the small intestine mucosa appears to be both a consequence of the alteration of the CCR6–CCL20 chemotactic axis and a cause of it because of the contrasting roles of IL-17 and IL-10/TGF-β1 on CCL20 production by enterocytes. A lack of responsiveness of the enterocytes to IL-17A could also be involved as we found that IL-17RA expression was decreased in treated HIV-1-infected individuals. IL-17A alone, but most markedly in synergy with proinflammatory cytokines such as TNF-α, induces the production of several inflammatory mediators via IL-17RA signaling.34, 40, 41 IL-21 promotes the differentiation of Th17 cells and induces IL-17RA expression,54 and treatment with IL-21 has been shown to improve gut Th17 restoration in treated SIV-infected macaques.55, 56 In contrast, Th1 cytokines, notably IFN-γ and IL-2, negatively regulate Th17 differentiation and IL-17RA expression.54, 57, 58 Our preliminary results revealed increased frequencies of Th1 cells in the small intestine mucosa of treated HIV-1-infected subjects that may contribute to the reduce IL-17RA expression. The mechanisms involved in, and the consequences of, the reduced IL-17RA expression by enterocytes in this setting deserves further studies.
In conclusion, we have described a mechanism that can explain why Th17 cells do not reconstitute efficiently in the small intestine mucosa of treated HIV-1-infected individuals despite sustained effective cART. The CCR6–CCL20 gut chemotactic axis is disrupted in this setting, leading to impaired homing of Th17, Th1Th17, and CCR6+ Treg cells to the small intestine mucosa that is only partly offset by the α4β7–MAdCAM-1 axis. In contrast, CCR6− Treg cells can efficiently migrate to the small intestine mucosa, possibly through CXCR3-driven chemotaxis, and this results in an altered Th17/CCR6− Treg ratio that blunts CCL20 production by the enterocytes, perpetuating a negative feedback loop for the recruitment of CCR6+CD4+ T cells to the small intestine mucosa in treated HIV-1-infected individuals.
METHODS
Subjects and samples
The ANRS EP44 study group has been previously described.22 Briefly, 20 HIV-1-infected individuals were recruited at the Toulouse University Hospital (Toulouse, France). They had been given sustained effective cART for a median of 5 years (IQR, 4–5.5), initiated at the chronic stage of infection (median nadir CD4+ T-cell count of 185 cells per μl, IQR 123–221 cells per μl). All had sustained plasma HIV-1 RNA <20 copies per ml. Their median CD4+ T-cell count was 668 cells per μl (IQR 451–849 cells per μl) on cART at the time of blood and gut sampling (clinical information is provided in Supplementary Table S1). Fourteen uninfected individuals were used as controls. The two groups were similar in terms of their age (51 vs. 53 years, P=0.44) and gender (male 86% vs. 67%, P=0.32). The frequency of CD4+ T cells remained lower in the peripheral blood of treated HIV-1-infected individuals than in uninfected controls (40.7% vs. 64.8%, P<0.0001) despite their being on sustained suppressive cART. Biopsies of duodenojejunal mucosa were taken from each patient during an upper endoscopy, and peripheral blood samples were taken at the same time. Certain analyses could not be performed on the entire study population because samples were unavailable for some subjects. All individuals were free of inflammatory or lymphoproliferative bowel disease on histopathologic examination.
Ethics statement
The study was approved by the Institutional Review Board CPP Sud-Ouest et Outre-Mer II. All participants provided informed consent (trial registration number NCT01038401).
Isolation of small intestine mucosal lymphocytes
Duodenojejunal biopsies (n=5) were digested with 0.5 mg ml−1 collagenase type II-S (Sigma-Aldrich, St Louis, MO). T lymphocytes were isolated by positive selection (EasySep Human CD3 Positive Selection Kit, Stemcell, Grenoble, France) and processed immediately.
Immunophenotyping of peripheral blood and mucosal lymphocytes
Flow cytometry analyses were performed on a BD LSRFortessa driven by the FACSDiva software (BD Biosciences, Franklin Lakes, NJ). Th17 (CD3+CD4+CXCR3−CCR4+CCR6+CD161+) and Th1Th17 (CD3+CD4+CXCR3+CCR4−CCR6+CD161+) cells were stained with anti-human CD3-BV655 (OKT3, Biolegend), CD4-PE-Cy7 (SK3), CXCR3-PerCP-Cy5.5 (1C6), CCR4-PE (1G1), CCR6-BV786 (11A9) (all from BD Biosciences, San Diego, CA), and CD161-eFluor450 (HP-3G10, eBiosciences, San Diego, CA) monoclonal antibodies (mAbs).
Treg cells were stained with anti-human CD3-PerCP-Cy5.5 (UCHT1, Ozyme, St Quentin Yvelines, France) or CD3-BV655 (OKT3, Biolegend), CD4-PacificBlue (RPA-T4, BD Biosciences), CD127-PE or FITC (R34.34, Beckman Coulter, Brea, CA), CD25-PE-Cy7 (M-A25), CD45RA-APC-Cy7 (HI 100), HLA-DR-PE-Cy5 (L243), CCR6-BV768 (11A9) (all from BD Biosciences), and FoxP3-AlexaFluor700 (PCH101, eBiosciences) mAbs using Cytofix/Cytoperm (BD Biosciences). CCR6 expression on IL-17- and IL-10-producing memory Treg cells was assessed by flow cytometry. Cells were activated with phorbol 12-myristate 13-acetate (20 ng ml−1) and ionomycin (1,000 ng ml−1) for 6 h and monensin was added after 1 h, before staining with anti IL-17-PE (eBio64DEC17, eBiosciences) and anti-IL-10-PerCP-Cy5.5 (JES3-0D7, Biolegend) mAbs using Cytofix/Cytoperm (BD Biosciences).
The cell subsets were also analyzed for the expression of the gut-homing receptors with anti-human CCR4-PE (1G1), CCR5-PerCP-Cy5.5 (2D7), CCR6-BV786 (11A9), CCR9-FITC (112509), CCR10-APC (314305), CXCR3-PerCP-Cy5.5 (1C6), CXCR4-PE-Cy5 (12G5), and β7-APC (FIB504) mAbs (all from BD Biosciences, except CCR9 and CCR10 mAbs from R&D Systems, Minneapolis, MN). We previously showed that β7+CD4+ T cells coexpress the α4 integrin chain, with Förster resonance energy transfer demonstration of the association between the α4 and β7 chains. β7+CD4+ T cells were also labeled with the α4β7 Act-1 mAb.22
Quantification of HIV-1 in the small intestine mucosa
HIV-1 DNA/RNA was quantified by real-time PCR/RT-PCR with primer pairs and Taqman probes specific for LTR/gag on a Light-Cycler (Roche Diagnostics, Meylan, France), as previously described.59, 60 HIV-1 DNA is expressed as copies per 106 gut CD4+ T cells. HIV-1 RNA is expressed as copies per mg of gut tissue.
Quantification of CCL20, IL-8, and IL-17RA mRNA in small intestine epithelial cells
RNA was extracted from small intestine epithelial cells using the RNeasy minikit (Qiagen, Hilden, Germany). The primers were designed to amplify a fragment encompassing a spliced region of CCL20, IL-8, and IL-17RA mRNA. RT-PCR was performed on a LightCycler 480 (Roche). Data are given as relative quantities of CCL20, IL-8, and IL-17RA mRNA normalized to GAPDH mRNA (ΔΔCt method). The primer and probe sequences used were: GAPDH sense, 5′-GGGTGTGAACCATGAGAAGT-3′, antisense, 5′- GACTGTGGTCATGAGTCCT-3′, and probe, 5′-CAGCAATGCCTCCTGCACCACCAA-3′; CCL20 sense, 5′-CTTTGATGTCAGTGCTGCTACT-3′, antisense, 5′-GATTTGCGCACACAGACAACT-3′, and probe, 5′-CACACGGCAGCTGGCCAATGAAGG-3′; IL-8 sense, 5′-CTTCCTGATTTCTGCAGCTC-3′, antisense, 5′-GGTGGAAAGGTTTGGAGTATG-3′, and probe, 5′-CACTCCTTGGCAAAACTGCACCTTC-3′; IL-17RA sense, 5′-GTGGCTCACATCGAATGGAC-3′, antisense, 5′-GCACAAACGTTCATTGGTGTTC-3′, and probe, 5′-TCGAGGTACAGGATGCTGGCGTCTGTC-3′.
Immunohistochemistry
Fresh tissues were fixed in 4% neutral buffered formalin and embedded in paraffin. Immunohistochemistry was performed on 3 μm sections using anti-CCL20 (MAB360, R&D Systems) and MAdCAM-1 (MCA2321T, Serotec, Kidlington, Oxfordshire, UK) mAbs and the appropriate secondary antibodies. MAdCAM-1 expression was quantified on endothelial cells semiquantitatively. Quantification was performed using LAS v3.7 (Leica microsystems, Wetzlar, Germany) and NIS-element (Nikon, Tokyo, Japan) in a blinded manner regarding HIV-1 infection status.
Isolation and culture of primary small intestine epithelial cells
Human intestinal tissue was obtained from small bowel surgical resections of HIV-negative individuals at the department of general and digestive surgery of Toulouse University Hospital, France. All individuals were free of inflammatory bowel disease. Informed consent was obtained from each donor as approved by the local ethics committee. Intestinal tissue was digested using collagenase, dispase, and DNAse. The digests were subjected to Percoll density gradient and epithelial cells were isolated by magnetic positive selection (human epithelial cell adhesion molecule (EpCAM)-positive selection kit, Stemcell). Cell purity was >95% by flow cytometry (anti-EpCAM mAb, Stemcell). Cells were placed in gelatin-coated wells (Corning BioCoat, New York, NY) and cultured in Dulbecco’s modified Eagle’s medium/F-12 (1:1), 5% fetal bovine serum, and 1% penicillin-streptomycin-amphotericin B supplemented with hepatocyte culture medium (HCM) Single Quote kit (Lonza, Basel, Switzerland).
Polarized enterocyte cultures
Primary small intestine epithelial cells were cultured on transwell inserts (0.4 μm pore polycarbonate membranes coated with Corning Matrigel) at 4.105 cells per insert. They were cultured in Dulbecco’s modified Eagle’s medium/F-12 (1:1), 5% fetal bovine serum, 1% penicillin-streptomycin-amphotericin B with ITS (Sigma-Aldrich) and 5 ng ml−1 epidermal growth factor for 2 days. Fetal bovine serum was then removed from the medium, while hydrocortisone and 3,3′,5-triiodo-L-thyronine (Sigma-Aldrich) were added. This resulted in monolayers of polarized epithelial cells. The differentiation of the enterocytes was checked by their expression of CK-20 (mAb from Santa Cruz Biotechnology, Dallas, TX) and by the organization of tight junctions revealed by continuous ZO-1 staining (mAb from Invitrogen, Carlsbad, CA) on an inverted confocal laser scanning microscope (LSM 710, Zeiss, Oberkochen, Germany) (Supplementary Figure S2). The enterocytes also expressed CK-18 and EpCAM (data not shown). The integrity of the epithelium was checked by measuring the transepithelial electrical resistance between the apical and basolateral sides of the monolayer (mean: 450 ohms cm−2, as assessed using Millicell ERS-2, Millipore, Darmstadt, Germany).
Stimulation of primary enterocytes ex vivo
TLR ligands Pam3CSK4 (TLR1/2 agonist), heat-killed Listeria monocytogenes (HKLM; TLR2 agonist), lipopolysaccharide (LPS; TLR4 agonist), Salmonella typhimurium flagellin (ST-FLA; TLR5 agonist), FSL-1 (TLR6/2 agonist), and NLR ligand muramyl dipeptide (MDP; NOD2 agonist) were all purchased from InvivoGen (Toulouse, France). Pam3CSK4, LPS, ST-FLA, and FSL-1 were used at 0.1 or 1 μg ml−1; HKLM was used at 108 cells per ml; MDP was used at 1 or 10 μg ml−1. Human cytokines (IL-1β, TNF-α, IL-17A, IL-10, and TGF-β1 from eBiosciences) were used at 0.5, 5, or 50 ng ml−1. All stimulations were performed during 15 h on the differentiated enterocytes cultured on transwell inserts, without epidermal growth factor, hydrocortisone, or 3,3′,5-triiodo-L-thyronine in the medium. TNF-α and IL-1β were positive controls for CCL20 induction (data not shown).
Flow cytometry sorting and ex vivo culture of Treg and Th17 cells
CCR6+ and CCR6− Treg and Th17 cells were sorted from memory CD4+ T cells by flow cytometry on a FACSARIA II (BD Biosciences). Purities of sorted cells were >99% for all subsets. Sorted cells were stimulated by CD3/CD28 Dynabeads and cultured in OpTmizer medium (Life Technology, Carlsbad, CA) with IL-2 for 1–2 weeks.
Ex vivo functional profiles of Treg and Th17 cells
Flow cytometry-sorted Treg and Th17 cells were cultured ex vivo and activated with phorbol 12-myristate 13-acetate/ionomycin for 6 h. For coculture experiments, various proportions of Treg and Th17 cells were placed in the bottom chamber of the transwells for coculture with primary small intestine epithelial cells during 15 h. We assessed CCL20 production by quantifying CCL20 mRNA in the enterocytes by RT-PCR, and CCL20, IL-17, IL-10, and TGF-β1 secreted proteins by enzyme-linked immunosorbent assay (R&D Systems).
Statistical analyses
Quantitative variables are described by their medians and interquartile ranges, shown as box plots, and compared using the Wilcoxon rank-sum test. The Wilcoxon signed-rank test was used for matched pairs. Correlations were estimated by calculating Spearman’s rank correlation coefficients. All tests were two sided, and P values of ≤0.05 were considered statistically significant. Statistical analyses were performed with Stata 10.0 (College Station, TX).
References
Hooper, L.V. & Macpherson, A.J. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 10, 159–169 (2010).
Lim, S.G. et al. Loss of mucosal CD4 lymphocytes is an early feature of HIV infection. Clin. Exp. Immunol. 92, 448–454 (1993).
Schneider, T. et al. Loss of CD4 T lymphocytes in patients infected with human immunodeficiency virus type 1 is more pronounced in the duodenal mucosa than in the peripheral blood. Berlin Diarrhea/Wasting Syndrome Study Group. Gut 37, 524–529 (1995).
Guadalupe, M. et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 77, 11708–11717 (2003).
Brenchley, J.M. et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200, 749–759 (2004).
Mehandru, S. et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200, 761–770 (2004).
Raffatellu, M. et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat. Med. 14, 421–428 (2008).
Brenchley, J.M. et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood 112, 2826–2835 (2008).
Anton, P.A. et al. Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. AIDS 14, 1761–1765 (2000).
Poles, M.A., Elliott, J., Taing, P., Anton, P.A. & Chen, I.S. A preponderance of CCR5(+) CXCR4(+) mononuclear cells enhances gastrointestinal mucosal susceptibility to human immunodeficiency virus type 1 infection. J. Virol. 75, 8390–8399 (2001).
Arthos, J. et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat. Immunol. 9, 301–309 (2008).
Cicala, C. et al. The integrin alpha4beta7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proc. Natl. Acad. Sci. USA 106, 20877–20882 (2009).
Brenchley, J.M. Mucosal immunity in human and simian immunodeficiency lentivirus infections. Mucosal Immunol. 6, 657–665 (2013).
Brenchley, J.M. et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12, 1365–1371 (2006).
Sandler, N.G. et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J. Infect. Dis. 203, 780–790 (2011).
Chege, D. et al. Sigmoid Th17 populations, the HIV latent reservoir, and microbial translocation in men on long-term antiretroviral therapy. AIDS 25, 741–749 (2011).
Kim, C.J. et al. Mucosal Th17 cell function is altered during HIV infection and is an independent predictor of systemic immune activation. J. Immunol. 191, 2164–2173 (2013).
Favre, D. et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 5, e1000295 (2009).
Schuetz, A. et al. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog. 10, e1004543 (2014).
Kok, A. et al. Early initiation of combined antiretroviral therapy preserves immune function in the gut of HIV-infected patients. Mucosal Immunol. 8, 127–140 (2015).
Mehandru, S. et al. Lack of mucosal immune reconstitution during prolonged treatment of acute and early HIV-1 infection. PLoS Med. 3, e484 (2006).
Mavigner, M. et al. Altered CD4+ T cell homing to the gut impairs mucosal immune reconstitution in treated HIV-infected individuals. J. Clin. Invest. 122, 62–69 (2012).
Streeter, P.R., Berg, E.L., Rouse, B.T., Bargatze, R.F. & Butcher, E.C. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature 331, 41–46 (1988).
Kunkel, E.J. et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J. Exp. Med. 192, 761–768 (2000).
Papadakis, K.A. et al. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J. Immunol. 165, 5069–5076 (2000).
Johansson-Lindbom, B. et al. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J. Exp. Med. 198, 963–969 (2003).
Mora, J.R. et al. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature 424, 88–93 (2003).
Iwasaki, A. & Kelsall, B.L. Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3alpha, MIP-3beta, and secondary lymphoid organ chemokine. J. Exp. Med. 191, 1381–1394 (2000).
Cook, D.N. et al. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity 12, 495–503 (2000).
Annunziato, F. et al. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 204, 1849–1861 (2007).
Acosta-Rodriguez, E.V. et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 8, 639–646 (2007).
Yamazaki, T. et al. CCR6 regulates the migration of inflammatory and regulatory T cells. J. Immunol. 181, 8391–8401 (2008).
Esplugues, E. et al. Control of TH17 cells occurs in the small intestine. Nature 475, 514–518 (2011).
Izadpanah, A., Dwinell, M.B., Eckmann, L., Varki, N.M. & Kagnoff, M.F. Regulated MIP-3alpha/CCL20 production by human intestinal epithelium: mechanism for modulating mucosal immunity. Am. J. Physiol. Gastrointest. Liver Physiol. 280, G710–G719 (2001).
Sierro, F. et al. Flagellin stimulation of intestinal epithelial cells triggers CCL20-mediated migration of dendritic cells. Proc. Natl. Acad. Sci. USA 98, 13722–13727 (2001).
Ivanov, I.I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Cosmi, L. et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J. Exp. Med. 205, 1903–1916 (2008).
Kleinschek, M.A. et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J. Exp. Med. 206, 525–534 (2009).
Seddiki, N. et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 203, 1693–1700 (2006).
Awane, M., Andres, P.G., Li, D.J. & Reinecker, H.C. NF-kappa B-inducing kinase is a common mediator of IL-17-, TNF-alpha-, and IL-1 beta-induced chemokine promoter activation in intestinal epithelial cells. J. Immunol. 162, 5337–5344 (1999).
Minegishi, Y. et al. Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J. Exp. Med. 206, 1291–1301 (2009).
Fontaine, J., Poudrier, J. & Roger, M. Short communication: persistence of high blood levels of the chemokines CCL2, CCL19, and CCL20 during the course of HIV infection. AIDS Res. Hum. Retroviruses 27, 655–657 (2011).
Choi, Y.K., Fallert, B.A., Murphey-Corb, M.A. & Reinhart, T.A. Simian immunodeficiency virus dramatically alters expression of homeostatic chemokines and dendritic cell markers during infection in vivo. Blood 101, 1684–1691 (2003).
Choi, Y.K., Whelton, K.M., Mlechick, B., Murphey-Corb, M.A. & Reinhart, T.A. Productive infection of dendritic cells by simian immunodeficiency virus in macaque intestinal tissues. J. Pathol. 201, 616–628 (2003).
Gosselin, A. et al. Peripheral blood CCR4+CCR6+ and CXCR3+CCR6+CD4+ T cells are highly permissive to HIV-1 infection. J. Immunol. 184, 1604–1616 (2010).
Monteiro, P. et al. Memory CCR6+CD4+ T cells are preferential targets for productive HIV type 1 infection regardless of their expression of integrin beta7. J. Immunol. 186, 4618–4630 (2011).
El Hed, A. et al. Susceptibility of human Th17 cells to human immunodeficiency virus and their perturbation during infection. J. Infect. Dis. 201, 843–854 (2010).
Mowat, A.M. & Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 14, 667–685 (2014).
Koenen, H.J. et al. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood 112, 2340–2352 (2008).
Beriou, G. et al. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood 113, 4240–4249 (2009).
Voo, K.S. et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc. Natl. Acad. Sci. USA 106, 4793–4798 (2009).
Duhen, T., Duhen, R., Lanzavecchia, A., Sallusto, F. & Campbell, D.J. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood 119, 4430–4440 (2012).
Ayyoub, M. et al. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t. Proc. Natl. Acad. Sci. USA 106, 8635–8640 (2009).
Lindemann, M.J., Hu, Z., Benczik, M., Liu, K.D. & Gaffen, S.L. Differential regulation of the IL-17 receptor by gammac cytokines: inhibitory signaling by the phosphatidylinositol 3-kinase pathway. J. Biol. Chem. 283, 14100–14108 (2008).
Ortiz, A.M. et al. IL-21 and probiotic therapy improve Th17 frequencies, microbial translocation, and microbiome in ARV-treated, SIV-infected macaques. Mucosal Immunol. advance online publication, 19 August 2015; doi: 10.1038/mi.2015.75 (2015).
Micci, L. et al. Interleukin-21 combined with ART reduces inflammation and viral reservoir in SIV-infected macaques. J. Clin. Invest. 125, 4497–4513 (2015).
Harrington, L.E. et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6, 1123–1132 (2005).
Park, H. et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6, 1133–1141 (2005).
Delobel, P. et al. Naive T-cell depletion related to infection by X4 human immunodeficiency virus type 1 in poor immunological responders to highly active antiretroviral therapy. J. Virol. 80, 10229–10236 (2006).
Mavigner, M. et al. HIV-1 residual viremia correlates with persistent T-cell activation in poor immunological responders to combination antiretroviral therapy. PLoS One 4, e7658 (2009).
Acknowledgements
This project was supported by grants from the French National Agency for Research on AIDS and Viral Hepatitis and Sidaction. We are indebted to the patients who took part in this study. We also thank A.-L. Iscache, V. Duplan-Eche, and F.-E. L’Faqihi for their help in flow cytometry cell sorting; F. Capilla, C. Salon, and T. Alsaati for their help in immunohistochemistry; S. Allart and A. Canivet for their help in microscopy; and O. Parkes for checking the English text.This work was partly presented at the 22nd Conference on Retroviruses and Opportunistic Infections, Seattle, WA, 23–26 February 2015, Abstract 274.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Rights and permissions
About this article
Cite this article
Loiseau, C., Requena, M., Mavigner, M. et al. CCR6− regulatory T cells blunt the restoration of gut Th17 cells along the CCR6–CCL20 axis in treated HIV-1-infected individuals. Mucosal Immunol 9, 1137–1150 (2016). https://doi.org/10.1038/mi.2016.7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2016.7
This article is cited by
-
Th22 cells are efficiently recruited in the gut by CCL28 as an alternative to CCL20 but do not compensate for the loss of Th17 cells in treated HIV-1-infected individuals
Mucosal Immunology (2021)
-
Impact of HIV-ART on the restoration of Th17 and Treg cells in blood and female genital mucosa
Scientific Reports (2019)
-
The loss of CCR6+ and CD161+ CD4+ T-cell homeostasis contributes to disease progression in SIV-infected rhesus macaques
Mucosal Immunology (2017)
-
Diversity and functions of intestinal mononuclear phagocytes
Mucosal Immunology (2017)