Abstract
Inflammatory bowel disease (IBD) is due to an aberrant immune response toward luminal antigens, probably commensal bacteria, in genetically susceptible subjects and is also influenced by environmental factors. An imbalanced intestinal microbiota known as “dysbiosis,” characterized by an increased proportion of pro-inflammatory microorganisms and a decreased proportion of anti-inflammatory microorganisms, has been repeatedly observed in IBD and is now recognized as a key factor in the gut inflammatory process. Fecal microbiota transplantation (FMT) has gained interest as a novel treatment option in IBD. The goal of FMT in IBD is not only to correct the dysbiosis, but also to restore a normal dialog between the host immune system and the microbiota. Data are still scarce, but the results of the first studies suggest that FMT could be a promising therapy in IBD. More studies are needed to define the best indications, optimal timing, frequency, mode of delivery, and the optimal donor for each patient.
Similar content being viewed by others
Introduction
Although major progress has been achieved in recent years, the pathogenesis of inflammatory bowel disease (IBD) has not yet been fully elucidated. It is generally accepted that IBD is caused by an aberrant immune response toward luminal antigens, most likely commensal bacteria, in genetically susceptible subjects and is also under the influence of environmental factors.1 Moreover, an imbalanced intestinal microbiota known as “dysbiosis” has been repeatedly observed in IBD and is now recognized as a key factor in the gut inflammatory process.2, 3, 4, 5, 6, 7 This dysbiosis is notably characterized by an increased proportion of pro-inflammatory microorganisms and a decreased proportion of anti-inflammatory microorganisms. From a therapeutic point of view, the correction of this dysbiosis is thus an attractive approach. Until now, efficacy of microbiome-based therapies such as probiotics or antibiotics has been disappointing in IBD. Fecal microbiota transplantation (FMT) consists of the administration of fecal material from a donor into the intestinal tract of a recipient to change their microbiota composition and restore healthy conditions. FMT has been used for several years for the treatment of recurrent Clostridium difficile infection (CDI), but this treatment in CDI has only recently been proven to be efficient in a randomized control trial.8 Following this study, FMT is now being evaluated in several other microbiota-driven diseases and particularly in IBD. CDI is a pure ecological problem characterized by a defect in gut microbiota barrier properties.9, 10 IBD, however, is far more complex and involves a deregulation of the host-microbes cross talk. The goal of FMT in IBD is thus not only to correct the dysbiosis, but also to restore a normal dialog between the host immune system and the microbiota. Data are still scarce, but the results of the first studies suggest a complex effect of FMT in IBD. In this paper, we will review the data currently available on FMT and IBD, and discuss the various and unresolved issues.
FMT and IBD: state of the art
FMT in ulcerative colitis
There are several studies and case reports on the use of FMT in ulcerative colitis (UC), but only two randomized control studies published to date. Angelberger et al.11 reported the results of FMT in a small group of five patients with moderate to severe UC who received FMT via nasojejunal tube and enema. None of the patients achieved remission by week 12, but a positive response was observed in one patient who showed an improvement in the Mayo score. In a small pilot study in four pediatric UC patients, Suskind et al.12 observed no clinical or biological improvement after a single FMT via nasogastric tube. Cui et al.13 reported the results of a prospective observational study in 15 patients with moderate to severe steroid-dependent UC. FMT was administered by upper endoscopy. They developed an original step-up FMT strategy where patients who failed to benefit from the initial FMT received a second treatment after 1 week. Eight patients (57%) responded to FMT with clinical improvement and discontinuation of steroids, five of whom received only one FMT. Four of the eight responders maintained long-term remission. Kunde et al.14 performed FMT in a small group of pediatric UC patients receiving enemas once a day for 5 days. Seven of the nine patients showed clinical remission at 1 week and six of them maintained clinical remission at 1 month. A recent meta-analysis of nine studies (including 122 patients, 79 with UC, 39 CD, and 4 IBD unclassified) described a pooled proportion of patients who achieved clinical remission of 36.2%.15 Among studies that included only UC patients, the proportion of patients achieving remission was 22%. There are only very limited data on FMT in pouchitis, with very few patients and disappointing results for most of them.16, 17
There are only two randomized controlled studies evaluating the efficacy of FMT in UC. Moayyedi et al.18 reported the results of a randomized, placebo-controlled trial using FMT to induce remission in patients with mild to moderate UC. Patients were randomized to receive FMT, or placebo, via enema once a week for 6 weeks. Results showed that subjects receiving FMT achieved remission significantly more often than those receiving placebo (9/38 vs. 2/37, P=0.03). The second trial was conducted by Rossen et al.19 in 50 patients with mild to severe UC. Fecal transplant was administered via nasoduodenal tube at week 0 and 3 with feces from healthy donors or autologous fecal microbiota. There was no statistically significant difference between the two groups regarding clinical and endoscopic remission. The results of these two randomized control trials may be influenced by inconsistent study designs, including differences in the control groups, dose and preparation of donor feces, delivery method, and frequency of FMT.
Compared with the impressive results of FMT in recurrent CDI (between 80 and 95% efficacy), these results in UC are disappointing. However, studies are still sparse with few patients and heterogeneous design, making comparison and conclusion difficult. Nevertheless, these data highlight the fact that the mechanism of action of FMT in IBD is radically different from that in CDI and suggest that results obtained in CDI will not be directly transposable in IBD.
FMT in Crohn’s disease
Less data are available for FMT in Crohn’s disease (CD) and, to our knowledge, no randomized controlled trial results have been published to date. Results reported in isolated cases or small series are heterogeneous, but suggest a positive effect of FMT in CD in some conditions.15, 20 In a meta-analysis, subgroup analysis demonstrated a pooled estimate of clinical remission of 60.5% in CD patients.15 In an open-label uncontrolled study, Suskind et al.21 reported the effect of FMT in nine pediatric patients (12–19 years) with mild to moderate active CD. Patients received FMT prepared from their parents’ stool by nasogastric tube with follow-up evaluations at 2, 6, and 12 weeks. Seven out of the nine patients were in clinical remission at week 2, with a decrease in disease activity score (PCDAI), C-reactive protein level, and calprotectin. However, the effect was transient as only five patients remained in remission at week 12. A prospective pilot study by Cui et al.22 enrolled 30 patients with active CD who received FMT through mid-gut administration in conjunction with treatment with mesalazine. After FMT, the overall clinical improvement and clinical remission rates at 1 month were 86.7 and 76.7%, respectively. The follow-up (after 15 months) showed sustained clinical remission with improvement in biological markers. Although these studies were uncontrolled, the results are impressive. Randomized placebo-controlled trials are needed to draw a clear picture of the potential effect of FMT in CD.
The first data on FMT in IBD suggest a positive effect. However, its magnitude and the way to optimize it remain to be clarified. Only larger randomized controlled studies will be able to address these questions.
How to optimize FMT in IBD?
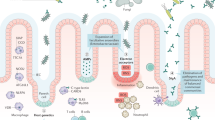
The efficacy of FMT in a patient with IBD is likely to be affected by many factors: patient status and preparation, donor selection, stool processing, and transplant delivery method.23 In addition, many questions regarding safety remain unanswered (Figure 1).
Unresolved question regarding FMT in IBD.
Selection of the donor, safety, and efficacy
Safety of FMT
Although FMT is perceived as “natural” or even “organic” by many patients and physicians, it has potential side effects. Using feces from a healthy donor presents potential risk of contracting a disease that can be spread through fecal material. Transmission of enteric pathogens via FMT is an important concern, but appears to be rare owing to the current screening procedure of donors.
Diverse adverse effects have been reported with FMT specifically in IBD, most of them being mild fever and mild gastrointestinal symptoms. IBD flares following FMT have been described both in UC and CD.24, 25 The route of FMT administration seems to modify the adverse effect profile. Fever and C-reactive protein rise have been described with nasojejunal route, whereas administration via the rectal route appears to be safer. Rare cases of aspiration pneumonia has been described in FMT via the nasojejunal route.26, 27 Mortality reported in the literature was not attributed to the FMT procedure but to patients’ comorbidities.20, 23 Advert metabolic response with important weight gain was reported in a woman who received FMT from an overweight donor. This case must however be interpreted with caution as the recipient was also overweight (body mass index 26) at the time of FMT.28
Screening of fecal donors before FMT may be just as necessary as screening organ donors to ensure that unwanted traits are not transferred with the transplant. In the literature, donors were either close relatives, household members, or unrelated (defined as healthy voluntary donors not related to the patient). Donors should be screened for social behaviors that could increase risk of transmitting infection and should be free of diseases that may be transmissible by stool.23
Efficacy of the FMT depends on the donor microbiota composition
Each individual has a distinct fecal microbiota composition, representing major difficulties in obtaining homogenous FMT treatment to test. Indeed, each FMT can be considered as donor-specific leading to donor-dependant effects. This microbiota diversity may account for some of the variability in the outcomes of different studies explaining the heterogeneity of the results.
Intrinsic characteristics of the donor’s microbiota could have a major role in the outcome of FMT. This is highlighted in the study by Moayyedi et al.18 Patients who received donor B’s feces were seen to achieve remission more often than others. Kelly et al.23 suggested that the efficacy of FMT may be related to the richness of a donors stool, and that one donor’s stool may be more “rich” than another. Indeed, the microbiota of donor B was enriched in members of the Lachnospiraceae family and Ruminococcus genus.29 Vermeire et al.26 investigated FMT as a therapeutic option in 14 patients with IBD. They observed that stools from donors that resulted in a successful FMT were characterized by a significantly higher bacterial richness.30 Richness of the donor microbiota is thus an important parameter to take into consideration. Another potentially important aspect to investigate in FMT is the amount of anti-inflammatory bacteria such as Faecalibacterum prausnitzii or bacteria producing short-chain fatty acid.31, 32 Short-chain fatty acid represent the major carbon flux from the diet through the gut microbiota to the host, and evidence is emerging for a regulatory role of short-chain fatty acid in local, intermediary, and peripheral metabolism.32 FMT enriched in short chain fatty acids producers might be of importance for the recipient, and the sustained and durable implantation of bacteria given by the FMT.
Beyond characteristics of donor microbiota, it is possible that combination of donor and recipient features might influence FMT efficacy in IBD, as human leukocyte antigen system does in tissue or bone marrow transplantation. These factors could be host- or microbiota-driven and could include genetics, microbiota, and diet. For example, the transfer of microbiota across gender has been identified as a possible issue. Studies have demonstrated a significant relationship between gut microbiome composition and sex, which may explain in part the sex-specific disparities in a variety of diseases such as autoimmune diseases.33 Despite these concerns, no published FMT study to date has age- or sex-matched donors with recipients, factors that may not affect short-term outcomes, but again may have consequence in the long-term follow-up, especially in pediatric patients.
A recent study of infants and children with healthy growth phenotypes living in Mirpur, Bangladesh, reported the 16S ribosomal RNA analysis of monthly fecal collection from birth up to the end of the second year of life.34 In this study, the ribosomal RNA analysis of bacterial components in the gut microbiota revealed 24 “age-discriminatory” taxa, whose changes in relative abundance over time define a program of normal maturation of the microbiota across biologically unrelated individuals. The authors developed two related metrics, relative microbiota maturity and microbiota-for-age Z-score that significantly correlated with the chronological age of children with healthy growth phenotypes. Then, applying their metrics on malnourished children in the same region, they showed that children with malnutrition have “immature” gut microbiota.34 The authors suggested that in healthy children, microbiota development is optimized to satisfy the different growth needs of the host at different ages. Hence, age-matched donors are more likely to be at a comparable level of microbiological and immunological maturity, and thus represent a more relevant source of material for pediatric populations.
Colonization success seems also to be different from one bacterium to another. Indeed, a donor strain belonging to a species already present in the recipient microbiota is more likely to establish in the recipient gut.35 There might thus be some donor-recipient microbiota compatibility factors to take into account to maximize the FMT effect.
The recent development of stool banks in several countries could help to facilitate FMT activities.36 The Microbiome Health Research Institute, d.b.a.OpenBiome, is a nonprofit organization dedicated to expanding safe access to fecal microbiota transplants, and to catalyzing research into the human microbiome. OpenBiome has built an international public stool bank that has two main objectives. First, donor stools are screened and processed in a standardized manner with the goal of facilitating safe FMTs, and second, they offer a platform for investigating other microbiome-associated diseases.
Donor selection is a major challenge for FMT in IBD and being able to identify a “good” donor is of high importance. There is a large volume of research available on the gut microbiota which serves as useful to narrow down donor selection. However, only large trial results will allow us to identify relevant criteria to select donors.
Preparation of the fecal microbiota transplant
Prior to FMT, fecal material has to be diluted and homogenized, so that it can be easily administered. According to many protocols, freshly produced donor stool (50–300 g) is dissolved in sterile saline water and used preferably within 6 h after emission. Water and other diluents (e.g., yogurt or milk) have also been described as a vehicle, with a trend toward improved outcome using larger volumes of prepared solution.37
Almost all fecal preparations are processed in an aerobic environment. In most publications, feces are infused as quickly as possible following the production by the donor. The exposure of fecal microbiota to aerobic conditions, even briefly, could be detrimental to anaerobes and conversely favor aerobes, with potential consequences to FMT outcome. The fact that autologous FMT led to a change in microbiota in the trial by Rossen et al.19 suggests that the microbiota was altered by fecal processing and thus supports this hypothesis. The majority of the beneficial bacteria in the gut are strict anaerobes, thus it is possible that preparing the transplant under anaerobic conditions could lead to better results.
For many techniques, logistical issues associated with preparation and use of fresh material may represent a limitation. To combat this, FMT using frozen material has recently been developed, thus reducing the barriers presented by the number and frequency of donors, screening, and the cost of such techniques. Frozen material is easier to use for both patients and physicians, and as there is a larger pool of samples to choose from, it allows the selection of the best matched donor. The standardization of donor material preparation using frozen feces will significantly simplify the clinical practice of FMT.38 Metagenomics studies highlight the role of storage conditions in maintaining intestinal microbiota integrity.39 The storage temperature (−20 or −80 °C) interferes in the conservation of DNA and freeze thaw is known to impact microbiota. Lee C et al.40 conducted the first randomized controlled trial comparing fresh vs. frozen FMT in recurrent CDI. FMT was given by enema, and findings for frozen FMT and fresh FMT were similar in terms of efficacy and safety. A non-randomized study using oral administration of capsules containing frozen FMT also showed good results in terms of efficacy in CDI.41 However, the expected effects of FMT in IBD are very different from those seen in recurrent CDI and therefore it is probable that the results seen in CDI cannot be extrapolated to IBD.
As well as differences in fecal microbiota preparation techniques, the procedure for preparing the recipient for transplant of the new microbiota also varies. Most of the authors use a classical colon cleansing with polyethylene glycol,19 but others use large spectrum antibiotics11 or no preparation at all.18 Although it seems logical to make some space for the new microbiota before administrating it, the best way to do so has not been directly studied and thus remains an open question.
The optimum preparation of donor stool has not yet been determined and there is currently high heterogeneity from one study to another: fresh vs. frozen stools; storage at −20 vs. −80 °C; use of cryopreservative or not, etc. Further studies are needed to identify the best approach and to set up a standard. Development of stool banks will help to reinforce homogeneity.36
Route of administration
The route of delivery represents another tricky matter. As with donor stool preparation, there is no clear consensus on the best method of instillation.
Many different ways of administration of the new microbiota have been described and tested. FMT can be delivered via the upper or the lower gastrointestinal tract. By the upper digestive tract, suspension of healthy donor stool is administered using a tube via the stomach, duodenum, or jejunum, or with oral ingestion of gelatin-coated capsules or frozen capsules. By the lower digestive tract, FMT is given directly via the endoscope channel into the terminal ileum, cecum, or sigmoid, or using rectal enema.
The upper gastrointestinal route might render the active constituent of FMT ineffective by the time it reaches the diseased colon. Conversely, in patients with extended colitis, rectal enema might be insufficient to induce a reset of microbiota in the entire colon. Gastric juice can damage Bacteroidetes, whereas some Firmicutes need to pass through the upper gastrointestinal tract to be activated. A lower gastrointestinal route of administration appears to achieve better outcomes than upper in CDI.42 It is likely that the choice of route of administration will depend on the microbes deemed important to be infused and on the disease being treated.
The number of infusions may be also critical. A single administration may be adequate for CDI, but not for chronic diseases such as IBD. It seems logical that, in IBD, the dysbiosis cannot be definitively corrected after only one FMT. It is possible that there is a dose response in FMT, and the scheme and number of infusions might be important. Although the microbiota has an effect on the host, it is also known that the host shapes the microbiota. Indeed, it is clear that host genes and diet are able to profoundly modify the microbiota,42, 43, 44 suggesting that FMT could only have a transient effect in IBD.
Timing of FMT
One important question is the timing of FMT administration during the course of the disease. First, at what point during the natural disease history should FMT be proposed? Data from Moayyedi et al.18 suggest that newly diagnosed UC patients may have the best outcome with FMT, suggesting a potential window of opportunity to treat patients with FMT early after diagnosis. The perturbation in the intestinal homeostasis might be more easily restored early in the course of the disease.
It is also important to consider whether or not flare is the best time to perform FMT. It is known that inflammation by itself is a major driver of dysbiosis,45 therefore, performing FMT during active gut inflammation might result in only a transient effect, as the infused microbiota will be immediately altered by the recipient’s inflammatory status. Moreover, the administration of a massive amount of microbial antigens on an inflamed and permeable mucosa may have, like adding fuel on the fire, some detrimental effect on the inflammatory process itself and could cause potential side effects such as microbial translocation. Therefore, a good treatment strategy might be to perform FMT in a patient who has achieved remission through conventional treatment.
Long-term safety of FMT
The greater concern about FMT relates to long-term safety. Risks include possible transmission of infectious agents (including unknown ones) or the development of diseases related to changes in the gut microbiota. The gut microbiota is a complex consortium with many poorly characterized components. The impact of transferring these complex communities from one person to another is not known. Studies in mice indicate that the composition of gut microbiota can affect host susceptibility to disease. Some groups have reported transferring colitis phenotypes from different knockout mouse models to wild-type mice.46, 47 In humans also, transfer of complex microbiota can modify the phenotypic expression of disease.48 Nevertheless, transmission of microbiota-driven disease in mice has been mostly shown in germ-free recipients and the effect of FMT in humans has been shown to be transient.
Long-term follow-up of patients receiving FMT is mandatory to answer the questions concerning safety and future adverse events.
Conclusion
FMT is a promising therapy in IBD, but more studies are needed to define the best indications, optimal timing, frequency, mode of delivery, and the optimal donor for each patient. Moreover, large studies will present the opportunity to identify active components of the gut microbiota that could then be isolated and used as potential therapeutic agents. Using this type of clinical-based rational selection process, we identified Faecalibacterium prausnitzii as a key player in intestinal homeostasis with therapeutic potential in IBD.31 Anti-inflammatory molecules produced by this bacterium have been recently identified.49, 50 In the future, gut-derived bacteria, used either alone as next-generation probiotics or in combination as artificial microbiota, could be used to counterbalance dysbiosis in a more controlled and standardized way compared with FMT.
Although IBD pathogenesis is related to an abnormal host-microbiota cross talk, the only therapeutic strategy envisaged until recently was to inhibit the overactivated immune system. With the current rise of microbiota-derived therapeutic approaches, a similar mistake should not be done. The host immune response and the microbiota are two players of the disease pathogenesis that should be taken into account together and probably targeted simultaneously to achieve optimal results.
References
Khor, B., Gardet, A. & Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 474, 307–317 (2011).
Sokol, H. & Seksik, P. The intestinal microbiota in inflammatory bowel diseases: time to connect with the host. Curr. Opin. Gastroenterol. 26, 327–331 (2010).
Sokol, H. et al. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm. Bowel Dis. 15, 1183–1189 (2009).
Manichanh, C. et al. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut 55, 205–211 (2006).
Mangin, I. et al. Molecular inventory of faecal microflora in patients with Crohn's disease. FEMS Microbiol. Ecol. 50, 25–36 (2004).
Gevers, D. et al. The treatment-naive microbiome in new-onset Crohn's disease. Cell Host Microbe 15, 382–392 (2014).
Morgan, X.C. et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 13, R79 (2012).
van Nood, E. et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 368, 407–415 (2013).
Leffler, D.A. & Lamont, J.T. Clostridium difficile infection. N. Engl. J. Med. 373, 287–288 (2015).
Gupta, S., Allen-Vercoe, E. & Petrof, E.O. Fecal microbiota transplantation: in perspective. Therap. Adv. Gastroenterol. 9, 229–239 (2016).
Angelberger, S. et al. Temporal bacterial community dynamics vary among ulcerative colitis patients after fecal microbiota transplantation. Am. J. Gastroenterol. 108, 1620–1630 (2013).
Suskind, D.L., Singh, N., Nielson, H. & Wahbeh, G. Fecal microbial transplant via nasogastric tube for active pediatric ulcerative colitis. J. Pediatr. Gastroenterol. Nutr. 60, 27–29 (2015).
Cui, B. et al. Step-up fecal microbiota transplantation strategy: a pilot study for steroid-dependent ulcerative colitis. J. Transl. Med. 13, 298 (2015).
Kunde, S. et al. Safety, tolerability, and clinical response after fecal transplantation in children and young adults with ulcerative colitis. J. Pediatr. Gastroenterol. Nutr. 56, 597–601 (2013).
Colman, R.J. & Rubin, D.T. Fecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic review and meta-analysis. J. Crohns Colitis 8, 1569–1581 (2014).
Landy, J. et al. Variable alterations of the microbiota, without metabolic or immunological change, following faecal microbiota transplantation in patients with chronic pouchitis. Sci. Rep. 5, 12955 (2015).
Fang, S. et al. Successful treatment of chronic Pouchitis utilizing fecal microbiota transplantation (FMT): a case report. Int. J. Colorectal Dis. 31, 1093–1094 (2016).
Moayyedi, P. et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 149, 102–109.e106 (2015).
Rossen, N.G. et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology 149, 110–118 e114 (2015).
Sha, S. et al. Systematic review: faecal microbiota transplantation therapy for digestive and nondigestive disorders in adults and children. Aliment. Pharmacol. Ther. 39, 1003–1032 (2014).
Suskind, D.L. et al. Fecal microbial transplant effect on clinical outcomes and fecal microbiome in active Crohn's disease. Inflamm. Bowel Dis. 21, 556–563 (2015).
Cui, B. et al. Fecal microbiota transplantation through mid-gut for refractory Crohn's disease: safety, feasibility, and efficacy trial results. J. Gastroenterol. Hepatol. 30, 51–58 (2015).
Kelly, C.R. et al. Update on fecal microbiota transplantation 2015: indications, methodologies, mechanisms, and outlook. Gastroenterology 149, 223–237 (2015).
De Leon, L.M., Watson, J.B. & Kelly, C.R. Transient flare of ulcerative colitis after fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin. Gastroenterol. Hepatol. 11, 1036–1038 (2013).
Khoruts, A. et al. Inflammatory bowel disease affects the outcome of fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin. Gastroenterol. Hepatol. (2016) (in press).
Vermeire, S. et al. Donor species richness determines faecal microbiota transplantation success in inflammatory bowel disease. J. Crohns Colitis 10, 387–394 (2016).
Baxter, M., Ahmad, T., Colville, A. & Sheridan, R. Fatal aspiration pneumonia as a complication of fecal microbiota transplant. Clin. Infect. Dis. 61, 136–137 (2015).
Alang, N. & Kelly, C.R. Weight gain after fecal microbiota transplantation. Open Forum Infect. Dis. 2, ofv004 (2015).
Grinspan, A.M. & Kelly, C.R. Fecal microbiota transplantation for ulcerative colitis: not just yet. Gastroenterology 149, 15–18 (2015).
Sokol, H. Toward rational donor selection in faecal microbiota transplantation for IBD. J. Crohns Colitis 10, 375–376 (2016).
Sokol, H. et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 105, 16731–16736 (2008).
Morrison, D.J. & Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 7, 189–200 (2016).
Ding, T. & Schloss, P.D. Dynamics and associations of microbial community types across the human body. Nature 509, 357–360 (2014).
Subramanian, S. et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 510, 417–421 (2014).
Li, S.S. et al. Durable coexistence of donor and recipient strains after fecal microbiota transplantation. Science 352, 586–589 (2016).
Kazerouni, A., Burgess, J., Burns, L.J. & Wein, L.M. Optimal screening and donor management in a public stool bank. Microbiome 3, 75 (2015).
Gough, E., Shaikh, H. & Manges, A.R. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin. Infect. Dis. 53, 994–1002 (2011).
Hamilton, M.J., Weingarden, A.R., Sadowsky, M.J. & Khoruts, A. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am. J. Gastroenterol. 107, 761–767 (2012).
Cardona, S. et al. Storage conditions of intestinal microbiota matter in metagenomic analysis. BMC Microbiol. 12, 158 (2012).
Lee, C.H. et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection: a randomized clinical trial. JAMA 315, 142–149 (2016).
Youngster, I. et al. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA 312, 1772–1778 (2014).
Kassam, Z., Lee, C.H., Yuan, Y. & Hunt, R.H. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am. J. Gastroenterol. 108, 500–508 (2013).
David, L.A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014).
Rawls, J.F., Mahowald, M.A., Ley, R.E. & Gordon, J.I. Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell 127, 423–433 (2006).
Winter, S.E. et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 339, 708–711 (2013).
Garrett, W.S. et al. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell 131, 33–45 (2007).
Elinav, E. et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145, 745–757 (2011).
Vrieze, A. et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143, 913–916.e917 (2012).
Quevrain, E. et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn's disease. Gut 65, 415–425 (2016).
Miquel, S. et al. Identification of metabolic signatures linked to anti-inflammatory effects of Faecalibacterium prausnitzii. MBio 6 (2015).
Acknowledgements
H.S. received consulting fee from Enterome, Maat pharma, Abbvie, Astellas, Takeda, MSD.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Pigneur, B., Sokol, H. Fecal microbiota transplantation in inflammatory bowel disease: the quest for the holy grail. Mucosal Immunol 9, 1360–1365 (2016). https://doi.org/10.1038/mi.2016.67
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2016.67
This article is cited by
-
Recipient factors in faecal microbiota transplantation: one stool does not fit all
Nature Reviews Gastroenterology & Hepatology (2021)
-
Role of melatonin in murine “restraint stress”-induced dysfunction of colonic microbiota
Journal of Microbiology (2021)
-
Short-Term Cohousing of Sick with Healthy or Treated Mice Alleviates the Inflammatory Response and Liver Damage
Inflammation (2021)
-
Fecal microbiota transplantation to maintain remission in Crohn’s disease: a pilot randomized controlled study
Microbiome (2020)
-
Integrated omics profiling of dextran sodium sulfate-induced colitic mice supplemented with Wolfberry (Lycium barbarum)
npj Science of Food (2020)