Abstract
Sublingual immunotherapy (SLIT) is a safe and efficient treatment for type 1 allergies; however, the underlying immunological mechanisms, particularly the phenotype of oral antigen-presenting cells (APCs) responsible for the induction of regulatory T (Treg) cells, remain unclear. We show here that the sublingual application of ovalbumin (OVA) induced antigen-specific Foxp3+ Treg cells in draining submandibular lymph nodes (ManLNs). Oral APCs were classified into macrophages, classical dendritic cells (cDCs), and Langerhans cells by flow cytometry. A major subset of oral cDCs with the CD103−CD11b+ phenotype showed retinoic acid (RA)-producing activity and converted naive CD4+ T cells to Foxp3+ Treg cells in a transforming growth factor-β- and RA-dependent manner in vitro. In the ManLNs, migratory CD103−CD11b+ cDCs also showed RA-producing activity. After the sublingual application of fluorescent OVA, fluorescence was detected in oral macrophages in tissues, followed by migratory CD103−CD11b+ cDCs in ManLNs and migratory CD103−CD11b+ cDCs were the main APCs responsible for the induction of sublingual antigen-specific Treg cells. The transfer of OVA-SLIT-induced Treg cells suppressed the OVA-induced hypersensitivity response. These results suggest that oral CD103−CD11b+ cDCs transport sublingual antigens to draining ManLNs and induce antigen-specific Foxp3+ Treg cells, and, thus, provide a rationale for developing cDC-based therapeutic approaches in SLIT.
Similar content being viewed by others
Introduction
Sublingual immunotherapy (SLIT) is an allergen-specific treatment for type 1 allergies, such as allergic rhinitis, with an improved safety profile and long-lasting effects.1, 2 SLIT operates by acting on the sublingual mucosa and increases allergen tolerance, possibly with the redirection of allergen-specific CD4+ T-cell responses from T-helper (Th) 2 to Th1 and the generation of regulatory T (Treg) cells.1, 2, 3 Treg cells are induced in the thymus and are also induced from naive CD4+ T cells in the periphery, and the transcriptional factor Foxp3 is required for the development and maintenance of the immunosuppressive functions of Treg cells.4 SLIT has been associated with increased numbers of Foxp3+ cells in biopsies from the sublingual mucosa5 and interleukin (IL)-10-producing Treg cells in peripheral blood samples.6, 7 Thus, a deeper understanding of the mechanisms underlying Treg-cell induction by SLIT may contribute to the development of new therapeutic strategies.
The oral cavity is colonized by a large number of commensal microbes and is constantly exposed to food antigens. However, acute allergic and inflammatory reactions occur relatively rarely at the oral mucosa.1 SLIT takes advantage of the pro-tolerogenic property of the oral mucosa for its beneficial effects and good safety profile; however, the underlying immunological mechanisms currently remain unclear. The oral mucosa consists of two main layers, the stratified epithelium and underlying lamina propria (LP), which are the equivalents of the epidermis and dermis of the skin, respectively.8 In the sublingual mucosa, four subsets of antigen-presenting cells (APCs) with distinct tissue distributions have been detected using immunohistochemistry and flow cytometry: (i) a major subpopulation of CD11b+CD11c− and CD11b+CD11c+ APCs in the LP, (ii) a minor subset of CD207 (langerin)+ Langerhans cells (LCs) located in the epithelium, and (iii) B220+120G8+ plasmacytoid dendritic cells (pDCs) found in the LP.9 Among these APCs, LCs and CD11b+CD11c− macrophage (Mφ)-like cells are considered to be critical for capturing sublingual antigens.9, 10, 11 A previous study suggested that oral CD11b+CD11c− Mφ-like cells presented sublingual antigens in draining lymph nodes (LNs) and promoted the differentiation of interferon-γ-producing Th1 cells and Foxp3+ Treg cells.11 However, the role of oral CD11c+ classical dendritic cells (cDCs) in tolerance induction has not yet been examined.8
In contrast, the mechanisms that underlie systemic tolerance to orally administered antigens, which is called “oral tolerance” and originates in the intestinal immune system, have been extensively investigated.12 In the LP of the intestines, most major histocompatibility complex II (MHCII)+CD11c+ dendritic cells (DCs) express CD103.13, 14 These CD103+ DCs are migratory cDCs in the LP. Intestinal CD103+ cDCs produce the vitamin A metabolite retinoic acid (RA), and intestinal CD103+ cDC-derived RA acts as a cofactor in the transforming growth factor (TGF)-β-mediated conversion of naive CD4+ T cells into Foxp3+ Treg cells.15, 16 Intestinal CD103+ cDCs express high levels of aldh1a2, the gene encoding retinal dehydrogenase (RALDH) 2,15 which is a key enzyme in the metabolic conversion of retinal to RA. Another population in the intestinal LP was shown to be CX3CR1high tissue-resident Mφs.13 CD64, the high-affinity IgG receptor FcγRI, was recently identified as a specific marker for Mφs, including intestinal CX3CR1high cells.17, 18
We recently reported that sublingual antigens were transported across sublingual ductal epithelial cells to oral APCs around the duct in mice.19 Therefore, we herein attempted to accurately identify and characterize oral APCs and clarify their roles in SLIT on the basis of established evidence regarding the properties of intestinal DCs. The results obtained revealed the previously unappreciated role of oral cDCs in Treg-cell induction and provide a rationale for developing cDC-based therapeutic approaches in SLIT.
Results
Sublingual administration of soluble protein antigen induces antigen-specific Foxp3+ Treg cells in draining ManLNs
It currently remains unclear whether SLIT, in effect, induces Foxp3+ Treg cells in vivo. In order to examine this, ovalbumin (OVA)-specific naive OT-II CD4+ T cells (CD45.2+) were adoptively transferred into CD45.1+ congenic mice, and the OVA protein was sublingually administered to the recipients on days 1 and 2. On day 5, the transferred OT-II T cells were detected in ManLNs, the draining LNs of the sublingual mucosa,20 and in non-draining auricular and mesenteric LNs (MesLNs), whereas the marked conversion of Foxp3+ cells was only detected in the draining ManLNs (Figure 1 a). The percentage of Foxp3+ cells among the transferred OT-II CD4+ T cells and total number of converted Foxp3+ Treg cells per mouse were significantly increased in the ManLNs only after the sublingual application of OVA (Figure 1 b, c). Furthermore, the transferred OT-II CD4+ T cells expressed interferon-γ, but not IL-4, IL-10, or IL-17A (Figure 1 d). These results clearly indicated that in addition to Th1 cells, SLIT was capable of inducing antigen-specific Foxp3+ Treg cells in draining ManLNs.
Sublingual antigen induces antigen-specific Foxp3+ Treg cells in ManLNs. Naive OT-II CD4+ T cells (CD45.2+) were adoptively transferred into CD45.1+ congenic mice, and OVA or vehicle was administered sublingually on days 1 and 2. On day 5, the conversion of Foxp3+ Treg cells in ManLNs, AurLNs, and MesLNs was analyzed by flow cytometry. (a) Representative plots with numbers indicating the percentage of gated cells. (b, c) Percentage of CD45.2+CD4+Vα2+ OT-II T cells expressing Foxp3 (b) and total number of CD45.2+CD4+Vα2+Foxp3+ OT-II T cells per mouse (c). Bars represent the mean±s.d. (n=3). *P<0.05 and **P<0.01 as determined by the Student’s t-test with Welch’s correction. (d) Intracellular cytokine staining of ManLN cells. Results are representative of three independent experiments. AurLN, auricular lymph node; IFN, interferon; IL, interleukin; ManLN, submandibular lymph node; MesLN, mesenteric lymph node; OVA, ovalbumin; Treg, regulatory T.
Classification of steady-state oral APCs by flow cytometry
Recent advances in defining mononuclear phagocytes led us to revisit the classification of oral APCs.14, 21, 22 We isolated cells from the lingual and sublingual tissues by enzymatic digestion using trypsin-EDTA followed by collagenase, and analyzed them by flow cytometry. By combining the expression of CD64 and CD11c, oral APCs were separated into CD64+ Mφs and MHCII+CD64−CD11c+ cells (Figure 2 a). The former consisted of MHCII+ and MHCII− cells, as recently described in the murine skin and heart.23, 24 The latter was subdivided into CD207+ and CD207− cells. CD207+ cells strongly expressed EpCAM (CD326) (Figure 2 a), as previously shown in epidermal LCs,25 and were not recovered by the treatment with collagenase alone (Figure 2 b) because collagenase does not have the ability to digest the epithelium. These results suggested that, among MHCII+CD64−CD11c+ cells, CD207+ cells are oral LCs that reside in the epithelium, while CD207− cells are oral cDCs in the LP. The absolute numbers showed that the predominant population comprised Mφs, followed by cDCs and then LCs in the tissues (Figure 2 c).
Oral APC subsets. (a, b) Lingual and sublingual tissues were either treated with trypsin-EDTA, followed by collagenase (a) or with collagenase alone (b) for flow cytometry. (c) Absolute cell number per mouse of each population in the tissues. Bars represent the mean±s.d. (n=4). *P<0.05 as determined by the Student’s t-test with Welch’s correction. Results are representative of three independent experiments. APC, antigen-presenting cell; cDC, classical dendritic cell; LC, Langerhans cell; MHCII, major histocompatibility complex II; Mφ, macrophage.
Flt3 ligand (Flt3L) has a key and non-redundant role in DC development, whereas Mφs and LCs develop independently of Flt3L.14, 21, 22 Therefore, in order to identify Flt3L-dependent oral DCs, mice were injected with B16 melanoma cells constitutively secreting Flt3L (B16-Flt3L cells) (Supplementary Figure S1a online), and when the tumor size reached 1–2 cm in diameter after 14–18 days, the number of APC subsets in the lingual and sublingual tissues were analyzed by flow cytometry. cDCs expanded more in B16-Flt3L-carrying mice than in control B16-carrying mice, irrespective of their expression of CD103 and CD11b (Supplementary Figure S1b , c, f). CD19−B220+PDCA-1+ pDCs, which were virtually absent in control mice, also significantly increased in B16-Flt3L-carrying mice; however, their total number was still low (Supplementary Figure S1d –f). In contrast, Mφs, LCs, and CD19+B220+ B cells did not expand in response to Flt3L. These results are consistent with our classification of oral APCs.
A recent study proposed that cDCs be subdivided into interferon regulatory factor (IRF) 8-dependent cDC1s and IRF4-dependent cDC2s, and also that CD172a (also known as signal regulatory protein α) may be used to identify cDC2s.21 Splenic CD8α+ cDCs (prototypical cDC1s) and a small number of oral CD103+CD11b− cDCs expressed IRF8 and low levels of CD172a (Figure 3). In contrast, splenic CD4+ cDCs (prototypical cDC2s) and oral CD103−CD11b+ cDCs highly expressed CD172a, but not IRF8. These results suggested that oral CD103+CD11b− and CD103−CD11b+ cDCs were classified as cDC1s and cDC2s, respectively.
Oral CD103−CD11b+ cDCs are phenotypically classified as cDC2s. Splenic (a) and oral (b) cDC subsets were analyzed for the expression of intracellular IRF8 and surface CD172a by flow cytometry. Black lines represent specific staining, and gray-shaded histograms represent isotype and unstained controls, respectively. The gating strategy is shown in the upper panels. Results are representative of three independent experiments. cDC, classical dendritic cell; IRF, interferon regulatory factor; MHCII, major histocompatibility complex II; Mφ, macrophage.
RA-producing oral CD103−CD11b+ cDCs induce Foxp3+ Treg cells in vitro
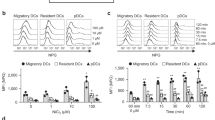
Intestinal CD103+ cDCs promote the de novo generation of Foxp3+ Treg cells via the production of RA.15, 16 Therefore, we used the ALDEFLUOR assay, which measures aldehyde dehydrogenase (ALDH) activity in cells, to identify oral tolerogenic APCs. Positioning of the ALDEFLUOR+ gate was based on an incubation with the specific inhibitor diethylaminobenzaldehyde. Splenic cDCs (both CD8α+ and CD4+ subsets) showed only marginal levels of ALDH activity (Supplementary Figure S2a). In the small intestinal (SI) LP, CD103+ cDCs (both CD11b+ and CD11b− subsets) expressed definitive ALDH activity (Supplementary Figure S2b). In lingual and sublingual tissues, the expression of ALDH activity was restricted to CD103−CD11b+ cDCs (Figure 4). These results suggest that oral CD103−CD11b+ cDCs are endowed with the ability to induce Treg cells.
Identification of RA-producing oral APCs. Cells isolated from the lingual and sublingual tissues were incubated with the ALDEFLUOR substrate in the presence or absence of DEAB, and analyzed by flow cytometry. (a) The gating strategy is shown. (b) CD45+MHCII+ALDEFLUOR+ cells were gated and analyzed for their surface marker expression. (c) Histograms represent the ALDEFLUOR fluorescence of the indicated subsets in the presence (gray shaded) or absence (black line) of DEAB. Results are representative of three independent experiments. APC, antigen-presenting cell; cDC, classical dendritic cell; DEAB, diethylaminobenzaldehyde; LC, Langerhans cell; MHCII, major histocompatibility complex II; Mφ, macrophage; RA, retinoic acid.
In order to examine this possibility, oral CD103−CD11b+ cDCs, LCs, and Mφs were purified and co-cultured with naive OT-II CD4+ T cells in the presence of the OVA323–339 peptide. Exogenous TGF-β and RA were not added to the culture. Five days after being incubated, oral CD103−CD11b+ cDCs induced Foxp3+ Treg cells at a significantly higher frequency than that induced by splenic cDCs, SI LP CD103+ cDCs, or oral LCs (Figure 5 a, b). In contrast, oral MHCII+ Mφs did not support the survival of naive CD4+ T cells. Furthermore, the induction of Foxp3+ Treg cells by oral CD103−CD11b+ cDCs was clearly inhibited by an anti-TGF-β blocking antibody and the synthetic RA receptor antagonists LE540 and LE135 (Figure 5 c, d). These results clearly indicated that oral CD103−CD11b+ cDCs were able to induce Foxp3+ Treg cells in a manner that was dependent on TGF-β and RA.
Oral CD103−CD11b+ cDCs induce Foxp3+ Treg-cell conversion in vitro in TGF-β- and RA-dependent manners. (a, b) Indicated APC subsets were co-cultured with CFSE-labeled naive OT-II CD4+ T cells in the presence of the OVA323–339 peptide. After 5 days of culture, CD4+ T cells were analyzed for CFSE dilution and Foxp3 expression by flow cytometry. (c, d) Control IgG (cIgG), neutralizing anti-TGF-β, or LE540 and LE135 (LE) were added to the culture medium, where indicated. The gating strategy for sorting APC subsets is shown in Figures 2a and 3 and Supplementary Figure S2b. Representative plots (a, c) and the percentage of CD4+ T cells expressing Foxp3 (b, d). Bars represent the mean±s.d. of triplicate (b) or quadruplicate (d) cultures. *P<0.05 and **P<0.01 as determined by a one-way analysis of variance followed by Tukey’s post hoc test. Results are representative of three independent experiments. APC, antigen-presenting cell; cDC, classical dendritic cell; CFSE, carboxyfluorescein succinimidyl ester; LC, Langerhans cell; LP, lamina propria; MHCII, major histocompatibility complex II; Mφ, macrophage; NS, not significant; OVA, ovalbumin; RA, retinoic acid; SI, small intestinal; TGF, transforming growth factor; Treg, regulatory T.
Oral CD103−CD11b+ cDCs express Treg-cell-inducing machinery
We searched for the migratory counterparts of oral CD103−CD11b+ cDCs in ManLNs, in which the presentation of sublingual antigens was expected. In LNs, lymphoid tissue-resident cDCs have been shown to express intermediate levels of MHCII and high levels of CD11c (MHCIIinterCD11chigh), whereas migratory cDCs and LCs express high levels of MHCII and intermediate to high levels of CD11c (MHCIIhigh CD11cinter to high).25, 26 Migratory cells were separated into CD207+EpCAM+ LCs as described,27 CD103+ cDCs, and CD103−CD11b+ cDCs (Figure 6 a). CD64+ Mφs were not detected in ManLNs.
Identification of RA-producing APCs in ManLNs. Cells isolated from ManLNs were incubated with the ALDEFLUOR substrate in the presence or absence of DEAB, and analyzed by flow cytometry. (a) The gating strategy is shown. (b) MHCII+ALDEFLUOR+ cells were gated and analyzed for their surface marker expression. (c) Histograms represent the ALDEFLUOR fluorescence of the indicated subsets in the presence (gray-shaded) or absence (black line) of DEAB. Results are representative of three independent experiments. APC, antigen-presenting cell; cDC, classical dendritic cell; DEAB, diethylaminobenzaldehyde; LC, Langerhans cell; ManLN, submandibular lymph node; MHCII, major histocompatibility complex II; RA, retinoic acid.
We next examined ALDEFLUOR+ cells in LNs. As previously reported,26 migratory CD103+ cDCs in MesLNs express high levels of ALDH activity (Supplementary Figure S3). We found that migratory CD103−CD11b+ cDCs in ManLNs also displayed higher levels of ALDH activity than those of migratory LCs and CD103+ DCs (Figure 6 b, c), as observed in the lingual and sublingual tissues (Figure 4).
Among the three RA-producing isoenzymes, the mRNA expression of Aldh1a2, but not Aldh1a1 or Aldh1a3 was significantly higher in SI LP CD103+ and oral CD103−CD11b+ cDCs than in splenic cDCs (Figure 7 a). Furthermore, migratory cDCs with these phenotypes also expressed Aldh1a2 mRNA in draining LNs, i.e., MesLNs and ManLNs (Figure 7 b). As splenic cDCs only exhibited marginal ALDH activity (Supplementary Figure S2a), these results indicated that RALDH2, which is encoded by Aldh1a2, was responsible for the RA-producing activity in SI LP CD103+ cDCs, oral CD103−CD11b+ cDCs, and their migratory counterparts in draining LNs.
Oral CD103−CD11b+ cDCs upregulate Aldh1a2 and Itgb8 expression upon migrating to ManLNs. APC subsets were isolated, using the gating strategy shown in Figures 3 and 6 a and Supplementary Figures S2b and S3a. The mRNA levels of Aldh1a1, Aldh1a2, and Aldh1a3 and Itgav, Itgb6, and Itgb8 in tissue (a) and LN (b) APC subsets indicated were determined by quantitative RT-PCR. Gene expression was normalized to Gapdh levels and expressed as a fold difference based on the value of splenic cDCs. Bars are the mean±s.d. (n=3). *P<0.05 and **P<0.01 as determined by a one-way analysis of variance followed by Tukey’s post hoc test. APC, antigen-presenting cell; cDC, classical dendritic cell; LC, Langerhans cell; LN, lymph node; LP, lamina propria; ManLN, submandibular lymph node; MesLN, mesenteric lymph node; MHCII, major histocompatibility complex II; Mφ, macrophage; ND, not detected; SI, small intestinal.
Intestinal CD103+ cDCs were previously reported to promote Foxp3+ Treg-cell induction via the integrin αvβ8-mediated activation of latent TGF-β;28, 29 therefore, the expression of integrin mRNAs was examined. In contrast to the broad expression of Itgav, Itgb8 was exclusively expressed in migratory CD103+ cDCs in MesLNs, and migratory CD103+ cDCs, CD103−CD11b+ cDCs, and LCs in ManLNs (Figure 7 b). The integrin αvβ6 is also known to activate latent TGF-β, and its expression was shown to be restricted to epithelial cells.30 Consistent with this finding, Itgb6 was rarely detected in any of the populations examined in the present study (Figure 7 a, b). These results suggest that integrin αvβ8 on migratory APCs is responsible for the activation of TGF-β in draining LNs.
Oral CD103−CD11b+ cDCs transport sublingual antigen to draining ManLNs and induce antigen-specific Foxp3+ Treg cells
We next attempted to identify the oral APC subsets that took up sublingually administered OVA in mice. After Alexa Fluor 488-labeled OVA was applied under the tongue, the labeled OVA was mainly taken up by CD64+ Mφs in the lingual and sublingual tissues; uptake peaked after 1–8 h and decreased by 16 h (Figure 8 a, b). In ManLNs, fluorescent cells were detected 8–16 h after the sublingual application of OVA, and the majority of fluorescent cells were migratory CD103−CD11b+ cDCs (Figure 8 c, d). These results suggested that the sublingual soluble protein antigen was transported to draining ManLNs by oral CD103−CD11b+ cDCs in spite of its efficient uptake by oral Mφs in the mucosa.
Oral CD103−CD11b+ cDCs transport sublingual antigen to ManLNs in spite of its efficient uptake by oral Mφs in the mucosa. Mice were left untreated (0 h) or sublingually treated with Alexa Fluor 488-labeled OVA for 1, 2, 4, 8, or 16 h. Lingual and sublingual tissues (a, b) and ManLNs (c, d) were then removed and processed for flow cytometry. Regarding the gating strategy, see Figures 2a and 6a. Plots pooled from three (a) or five (c) independent experiments with n=1 per experiment and the percentage (mean±s.d.) of Alexa Fluor 488+ cells in the indicated APC subsets at the indicated time points (b, d). APC, antigen-presenting cell; cDC, classical dendritic cell; LC, Langerhans cell; ManLN, submandibular lymph node; MHCII, major histocompatibility complex II; Mφ, macrophage; ND, not detected; OVA, ovalbumin.
We attempted to identify the APC subsets responsible for Foxp3+ Treg-cell induction in ManLNs by SLIT. In order to achieve this, resident cDCs, migratory LCs, migratory CD103+ cDCs, and migratory CD103−CD11b+ cDCs were purified from ManLNs 16 h after the sublingual application of OVA in mice, and were then co-cultured with naive OT-II CD4+ T cells ex vivo. Five days after being incubated, three migratory populations induced Foxp3+ cells (Figure 9 a). Although the percentages of the induced Foxp3+ Treg cells were similar among these migratory populations (Figure 9 b), their total number was significantly higher when stimulated with migratory CD103−CD11b+ cDCs (Figure 9 c). In addition, the total number of migratory CD103−CD11b+ cDCs was approximately threefold higher than that of migratory LCs or CD103+ cDCs in ManLNs (Supplementary Figure S4). These results suggested that migratory CD103−CD11b+ cDCs in ManLNs were the main APCs responsible for the induction of antigen-specific Foxp3+ Treg cells.
Sublingual antigen-primed migratory CD103−CD11b+ cDCs in ManLNs induce antigen-specific Foxp3+ Treg cells ex vivo. Mice were sublingually treated with OVA for 16 h, and ManLNs were then removed. Purified APC subsets from the ManLNs were co-cultured with CFSE-labeled naive OT-II CD4+ T cells. After 5 days of culture, CD4+ T cells were analyzed for CFSE dilution and Foxp3 expression by flow cytometry. The gating strategy for sorting APC subsets is shown in Figure 6a. Representative plots (a), the percentage of CD4+ T cells expressing Foxp3 (b), and the total number of converted CD4+Foxp3+ Treg cells (c). Bars represent the mean±s.d. of triplicate cultures. **P<0.01 as determined by a one-way analysis of variance followed by Tukey’s post hoc test. Results are representative of three independent experiments. APC, antigen-presenting cell; cDC, classical dendritic cell; CFSE, carboxyfluorescein succinimidyl ester; LC, Langerhans cell; ManLN, submandibular lymph node; NS, not significant; OVA, ovalbumin; Treg, regulatory T.
Suppression of hypersensitivity by SLIT-induced Treg cells
We determined whether SLIT-induced Treg cells are capable of suppressing hypersensitivity using the OVA-induced delayed-type hypersensitivity (DTH) model previously used to examine oral tolerance.31 The sublingual administration of the OVA antigen prior to sensitization significantly suppressed the DTH response (Figure 10 a). CD25+ Treg cells and CD25− non-Treg cells were purified from the ManLNs of mice sublingually administered OVA (Supplementary Figure S5), and then transferred to naive mice prior to sensitization with OVA. More than 95% of the Treg cells expressed Foxp3. Treg, but not non-Treg, cell transfer clearly suppressed the DTH response more than that in non-transferred mice (Figure 10 b). In addition, CD25+ Treg cells from vehicle control mice did not suppress this response. This result indicated that SLIT-induced antigen-specific Treg cells were responsible for tolerance to hypersensitivity.
SLIT-induced Treg cells suppress DTH. Mice were sublingually administered vehicle or OVA on days 0, 3, 7, 10, 14, and 17. (a) On day 21, mice were sensitized to OVA. On day 35, their ear pinnae were challenged with OVA. Ear swelling was measured at the indicated time points. Data are the mean±s.d. (n=8 ears). **P<0.01 as determined by a two-way analysis of variance followed by Bonferroni’s post hoc test. (b) On day 20, recipient mice were adoptively transferred or not with CD25+ or CD25− populations of donor ManLN cells as indicated. The recipient mice were sensitized and challenged as in (a). Ear swelling was measured 48 h after the challenge. Bars are the mean±s.d. (n=8 ears). **P<0.01 as determined by a one-way analysis of variance followed by Tukey’s post hoc test. Results are representative of three independent experiments. DTH, delayed-type hypersensitivity; ManLN, submandibular lymph node; NS, not significant; OVA, ovalbumin; SLIT, sublingual immunotherapy; Treg, regulatory T.
Discussion
We herein demonstrated that the sublingual application of an antigen induced antigen-specific Foxp3+ Treg cells in draining ManLNs, and that oral CD103−CD11b+ cDCs were responsible for Treg-cell induction. The sublingual administration of OVA induced Foxp3+ Treg cells in ManLNs, but not MesLNs, suggesting that although sublingual OVA may be swallowed, it was mostly absorbed into the sublingual mucosa and not, or to a much lesser extent, into the intestinal mucosa. ManLNs drain not only the oral mucosa, but also other head and neck regions, such as the nasolabial lymphatic plexus.32 Therefore, the gating strategy of ManLNs may include cDCs from other head and neck regions as well as the sublingual region. This study also showed that SLIT was able to induce not only Treg cells, but also Th1 cells. These results suggest that oral commensal bacteria direct T-cell polarization toward Th1 cells as well as Treg cells in a steady state oral environment.
We found that RA-producing cDCs were characterized by the CD103−CD11b+ phenotype in the oral mucosa, supporting the expression of CD103 not constituting a universal marker for RA-producing cDCs.26 Previous findings33 and the results of the present study showed that intestinal CD103− cDCs also exhibited RA-producing activity. Oral CD103−CD11b+ cDCs converted naive CD4+ T cells into Foxp3+ Treg cells in vitro in TGF-β- and RA-dependent manners, whereas oral MHCII+ Mφs did not exhibit this function. We did not add exogenous TGF-β in the conversion assay, in contrast to a previous study in which the addition of exogenous TGF-β induced the expression of Foxp3 by oral as well as intestinal Mφs.11 A previous study reported that CX3CR1+ Mφs induced the expansion of Treg cells in SI LP by producing IL-10.31 Therefore, oral Mφs may support Foxp3+ Treg-cell proliferation in sublingual LP, but not the survival of naive CD4+ T cells in vitro. Taken together, our results indicate that oral CD103−CD11b+ cDCs are potent inducers of Foxp3+ Treg cells, and this property may be associated with their ability to stimulate naive CD4+ T cells and the expression of the Treg-cell-inducing machinery, namely, RALDH2 and integrin αvβ8. In the intestines, the depletion of the CD103+CD11b+ or CD103+CD11b− cDC subsets did not affect the numbers of Foxp3+ Treg cells in the LP, and a reduction in Treg-cell numbers was only observed in animals lacking all CD103+ cDCs, suggesting that these two CD103+ cDC subsets contribute to the induction of tolerance.34 Therefore, intestinal CD103+ cDCs were used as a positive control in this study. Furthermore, in contrast to intestinal CD103+ cDCs, CD103+ cDCs in the oral mucosa and ManLNs were mainly CD11b−. Therefore, total CD103+ cDCs were herein investigated.
The present study showed that the frequency of ex vivo-induced Foxp3+ cells was similar among the three migratory subsets in ManLNs, i.e., CD207+ LCs, CD103+ cDCs, and CD103−CD11b+ cDCs. The expression of Itgb8 in these subsets indicates that these migratory subsets may possess the machinery to convert naive CD4+ T cells to Treg cells. However, the total number of Treg cells was significantly high when co-cultured with migratory CD103−CD11b+ cDCs, suggesting that this subset of DC may induce the better expansion and/or survival of Treg cells. This subset may also have presented higher doses of the sublingual antigen than the other subsets. In support of this, the sublingual fluorescent antigen was mostly detected in migratory CD103−CD11b+ cDCs in ManLNs. Fluorescence was almost not detected in migratory LCs or CD103+ cDCs despite their conversion of Treg cells ex vivo. This discrepancy may be explained as follows. Fluorescently undetectable levels of the antigen in APCs may serve to stimulate antigen-specific T-cell responses. The antigen may be transferred in a processed or undetectable form as suggested previously.35 This study also showed that the total number of migratory CD103−CD11b+ cDCs was approximately threefold higher than that of migratory LCs or CD103+ cDCs in ManLNs. These results strongly suggest that among migratory APCs, migratory CD103−CD11b+ cDCs are the main cell type responsible for the induction of antigen-specific Foxp3+ Treg cells in ManLNs. Resident cDCs in ManLNs induced Foxp3+ Treg-cell conversion to a lesser extent, and this phenomenon may be explained as above.
Our results showed that the residual cDC and LC population in the oral mucosa, which included CD103+ cDCs and LCs, did not exhibit ALDH activity, whereas migratory CD103+ cDCs in ManLNs did, but at a weaker level than that of migratory CD103−CD11b+ cDCs. The expression of Itgb8 also appeared to be extremely low in SI LP CD103+ cDCs and oral CD103−CD11b+ cDCs, whereas migratory CD103+ cDCs in MesLNs and the three migratory subsets in ManLNs strongly expressed Itgb8. These results suggest that RA-producing and/or TGF-β-activating abilities in these APC subsets were upregulated upon migration to the draining LNs.
This study showed that the sublingual antigen was captured by oral Mφs, but not cDCs or LCs in the mucosa, after 1–8 h and detected in migratory CD103−CD11b+ cDCs in ManLNs by 8–16 h. A previous study reported that, to induce oral tolerance, intestinal Mφs capture luminal OVA and transfer it to CD103+CD11b+ cDCs via a route involving gap junctions.35 Therefore, oral Mφs may have transferred the sublingual antigen to oral CD103−CD11b+ cDCs in the sublingual LP. Moreover, we recently reported, based on a histological analysis, that sublingual antigens, including OVA, were transported across sublingual ductal epithelial cells to oral APCs around the duct.19 Thus, we assumed that sublingual soluble protein antigens may enter the sublingual LP via the sublingual ductal epithelium as well as sublingual mucosal epithelium in order to be captured by oral Mφs in the LP.
The implantation of B16 cells genetically engineered to secrete Flt3L results in the massive expansion of DCs in vivo.36, 37, 38 As the injection of wild-type B16 cells into mice resulted in only minimal changes in peripheral blood counts and the splenocyte population,37 this protocol is commonly used to expand DCs in vivo, e.g., the small intestine.39, 40 In the present study, B16-carrying mice did not have an altered population of oral cDCs, LCs, and Mφs from that in wild-type mice (data not shown), and we consider oral cDCs and pDCs to have expanded in response to Flt3L in B16-Flt3L-carrying mice. A recent study strongly suggested that oral mucosal CD207+ cells in the stratified epithelium are genuine LCs.27 Furthermore, despite CD207+ cDCs being present in the dermis of the skin,25 the oral and vaginal mucosae appear to have no or very few CD207+ cDCs in the LP,27, 41 indicating that the oral CD207+ cells detected in this study are LCs, not cDCs. This study showed that oral LCs did not expand in B16-Flt3L-carrying mice, whereas a recent study reported a reduction in oral LCs in Flt3-deficient mice.27 This discrepancy may be explained by the limited space of the oral mucosal epithelium for the expansion of oral LCs in response to Flt3L.
In addition to the induction of Treg cells with TGF-β, RA also enhanced the expression of the integrin α4β7 and the chemokine receptor CCR9 on T cells upon activation and imprinted them with gut tropism.42 Furthermore, this study showed that SLIT-induced Treg cells were able to suppress the DTH response as reported in oral tolerance.31 Therefore, RA produced by cDCs in ManLNs may induce gut-homing receptors on antigen-specific Treg cells, and these Treg cells may, in turn, prevent hypersensitivity with the assistance of the intestinal immune system.
In conclusion, we herein revealed that oral CD103−CD11b+ cDCs possessed the necessary properties to induce Foxp3+ Treg cells, possibly in order to establish peripheral tolerance, i.e., SLIT. Many different subsets of cDCs in rodents and humans can be divided broadly on the basis of their requirement for transcription factors into cDC1 and cDC2,21 and our phenotypic characterization suggested that oral CD103−CD11b+ cDCs were classified into cDC2s. The gene expression profiles and functions of human CD1c+ cDCs resemble those of mouse cDC2s,21, 43 suggesting that human oral CD1c+ cDCs may be the functional homologue of mouse oral CD103−CD11b+ cDCs. Human CD1c+ cDCs have been commonly detected in biopsies from the sublingual mucosa.5 Therefore, targeting oral CD1c+ cDCs may serve the development of new therapeutic strategies in SLIT.
Methods
Mice. C57BL/6 (CD45.2+) mice were purchased from Japan SLC (Hamamatsu, Japan). Congenic C57BL/6 CD45.1+ mice were provided by the RIKEN BRC through the National Bio-Resource Project of the MEXT, Japan. OT-II mice have been described previously44 and were maintained on a C57BL/6 background (CD45.2+). All experimental protocols were approved by the Institutional Animal Care and Use Committee and by the Genetic Modification Safety Committee of Tohoku University (Sendai, Japan).
Sublingual administration. OVA (25 mg ml−1; Sigma–Aldrich, St Louis, MO) or Alexa Fluor 488-conjugated OVA (2.5 mg ml−1; Life Technologies, Carlsbad, CA) was applied under the tongues of mice in 20-μl aliquots of phosphate-buffered saline (PBS) containing 3% carboxymethyl cellulose under ketamine–xylazine (50/20 mg kg−1, intraperitoneally) anesthesia, as described previously.19
APC isolation. Mice were killed and then perfused with 20 ml of PBS through the left ventricle. The sublingual mucosae with tongues were excised by trimming off the roots of the tongues and cut into fragments. The tissues were incubated with 0.25% trypsin and 1 mM EDTA (trypsin-EDTA; Wako Pure Chemical, Osaka, Japan) for 1 h at 37 °C with shaking; this treatment was omitted in some experiments. After being washed with RPMI 1640 containing 10% fetal bovine serum, the tissues were further incubated with 1 mg ml−1 of collagenase (Wako Pure Chemical) and 0.1 mg ml−1 of DNase I (Sigma–Aldrich) in RPMI 1640 medium containing 10% fetal bovine serum, again for 1 h at 37 °C with shaking. Cells were then filtered, using a 70-μm cell strainer. SI LP cells were prepared, as described previously.45 Lingual and sublingual mucosal and SI LP cells were further purified by density gradient centrifugation with Lympholyte-M (Cedarlane, Hornby, BC, Canada), except when absolute APC numbers were determined by flow cytometry. Regarding spleen and LN cells, the spleens and LNs were cut into pieces and incubated with 1 mg ml−1 of collagenase D (Roche, Basel, Switzerland) and 0.1 mg ml−1 of DNase I in RPMI 1640 medium containing 10% fetal bovine serum for 30 min at 37 °C with shaking. Spleen cells were depleted of red blood cells by hypotonic lysis. In the in vitro and ex vivo Treg-cell conversion assays and quantitative RT-PCR analysis, APCs were sorted on a FACSAria II cell sorter (BD Biosciences, San Jose, CA).
Flow cytometry. Cells were isolated, as described in the previous sections. Non-specific staining was blocked with anti-CD16/32 (2.4G2; produced in-house). Antibodies used for cell staining are listed in Supplementary Table S1. Intracellular staining for Foxp3 and IRF8 was performed using a Foxp3 staining buffer set (eBioscience, San Diego, CA). Intracellular cytokines were stained as described previously.46 Dead cells were stained with either DAPI or Zombie Yellow (BioLegend, San Diego, CA) and excluded. Absolute cell numbers were calculated using CountBright absolute counting beads (Life Technologies). Data were acquired on an LSRFortessa cell analyzer (BD Biosciences), and analyzed using FlowJo software (Tree Star, Ashland, OR).
In vivo Treg-cell conversion assay. Naive CD4+ T cells (defined as CD4+NK-1.1−CD62LhighCD44lowCD25−) were purified from the spleens and LNs of OT-II mice (CD45.2+) using CD4+ T cell isolation kit II (Miltenyi Biotec, Bergish Gladbach, Germany), followed by a FACSAria II cell sorter. Purified cells (2 × 106) were injected intravenously into CD45.1+ mice. On days 1 and 2, OVA was applied under the tongue, as described in the section on sublingual administration. On day 5, LNs were harvested, and the expression of Foxp3 and intracellular cytokines by OT-II T cells was determined by flow cytometry.
Flt3L treatment in vivo. B16-Flt3L cells were provided by Dr A. Kumanogoh (Graduate School of Medicine, Osaka University, Osaka, Japan) and grown in RPMI 1640 medium with 10% fetal bovine serum. The concentration of Flt3L released into the medium was measured using the Quantikine ELISA Kit (R&D systems, Minneapolis, MN). A total of 1 × 106 cells were injected subcutaneously into each dorsal flank of mice. B16 cells (provided by the Cell Resource Center for Biomedical Research, Institute of Development, Aging and Cancer, Tohoku University) were used as a control.
ALDEFLUOR assay. The ALDH activity of cells was determined using the ALDEFLUOR kit (STEMCELL Technologies, Vancouver, BC, Canada) according to the manufacturer’s instructions. Briefly, cells (5 × 106 cells per ml) were incubated in the dark for 30 min at 37 °C in ALDEFLUOR assay buffer containing ALDEFLUOR substrate, with or without the ALDH inhibitor diethylaminobenzaldehyde. Cells were subsequently stained with antibodies and DAPI, and analyzed by flow cytometry.
In vitro and ex vivo Treg-cell conversion assay. Regarding carboxyfluorescein succinimidyl ester (CFSE) labeling, naive OT-II CD4+ T cells were treated in PBS containing 5 μM CFSE for 10 min at 37 °C. Cells were cultured in 200 μl of RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 1 mM sodium pyruvate, 50 μM 2-mercaptoethanol, and antibiotics. In the in vitro assay, 5 × 103 APCs were co-cultured with 5 × 104 CFSE-labeled naive OT-II CD4+ T cells in the presence of 100 nM OVA323–339 peptide (MBL, Nagoya, Japan), with or without LE540 (Wako Pure Chemical) and LE135 (Cayman Chemical, Ann Arbor, MI) (1 μM each), 10 μg ml−1 of anti-TGF-β (1D11; R&D systems), or control mouse IgG1 (MOPC-21; BioLegend). In the ex vivo assay, 2 × 104 APCs that were isolated from the ManLNs of mice 16 h after the sublingual administration of OVA were co-cultured with 2 × 105 CFSE-labeled naive OT-II CD4+ T cells. Mouse recombinant IL-2 (BioLegend) was added to culture wells at 5 ng ml−1 on days 2 and 4. On day 5, the dilution of CFSE and acquisition of Foxp3 by OT-II T cells were assessed by flow cytometry.
Quantitative RT-PCR. Isolated APCs (>4 × 104) were lysed in Isogen II (Nippon Gene, Tokyo, Japan), and total RNA was extracted, as described in the manufacturer’s instructions. cDNA was generated using the Transcriptor First Strand cDNA Synthesis Kit (Roche). Quantitative RT-PCR was performed using LightCycler Fast Start DNA Master SYBR Green I and the LightCycler 1.5 system (Roche). The primers used for quantitative RT-PCR are shown in Supplementary Table S2. Gene expression was normalized to Gapdh mRNA levels and expressed as relative units.
OVA-DTH. In order to sensitize mice to OVA, 150 μg OVA in a 100-μl emulsion of equal volumes of PBS and complete Freund’s adjuvant (Difco Laboratories, Detroit, MI) was injected subcutaneously into each dorsal flank of mice. Two weeks later, their ear pinnae were challenged intradermally with 50 μg OVA in 20 μl PBS. Ear swelling was measured as described previously.47
Treg-cell transfer. Donor ManLN cells were separated into CD25+ and CD25− populations using the EasySep Mouse CD25 Treg Positive Kit (STEMCELL Technologies). A total of 1 × 105 CD25+ Treg or 1 × 106 CD25− non-Treg cells were then transferred intravenously into recipient mice. The purity of transferred cell populations was evaluated by flow cytometry.
Statistical analysis. Experimental values are expressed as means±s.d. A statistical analysis was performed using Prism 6 software (GraphPad Software, San Diego, CA) as described in the figure legends. Values of P<0.05 were considered to indicate significance.
References
Novak, N., Bieber, T. & Allam, J.P. Immunological mechanisms of sublingual allergen-specific immunotherapy. Allergy 66, 733–739 (2011).
Calderon, M.A., Simons, F.E., Malling, H.J., Lockey, R.F., Moingeon, P. & Demoly, P. Sublingual allergen immunotherapy: mode of action and its relationship with the safety profile. Allergy 67, 302–311 (2012).
Akdis, M. & Akdis, C.A. Mechanisms of allergen-specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J. Allergy Clin. Immunol. 133, 621–631 (2014).
van Loosdregt, J. & Coffer, P.J. Post-translational modification networks regulating FOXP3 function. Trends Immunol. 35, 368–378 (2014).
Scadding, G.W. et al. Sublingual grass pollen immunotherapy is associated with increases in sublingual Foxp3-expressing cells and elevated allergen-specific immunoglobulin G4, immunoglobulin A and serum inhibitory activity for immunoglobulin E-facilitated allergen binding to B cells. Clin. Exp. Allergy 40, 598–606 (2010).
Bohle, B., Kinaciyan, T., Gerstmayr, M., Radakovics, A., Jahn-Schmid, B. & Ebner, C. Sublingual immunotherapy induces IL-10-producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation. J. Allergy Clin. Immunol. 120, 707–713 (2007).
Suarez-Fueyo, A. et al. Grass tablet sublingual immunotherapy downregulates the TH2 cytokine response followed by regulatory T-cell generation. J. Allergy Clin. Immunol. 133, 130–138.e1-2 (2014).
Hovav, A.H. Dendritic cells of the oral mucosa. Mucosal Immunol. 7, 27–37 (2014).
Mascarell, L. et al. Oral dendritic cells mediate antigen-specific tolerance by stimulating TH1 and regulatory CD4+ T cells. J. Allergy Clin. Immunol. 122, 603–609 e5 (2008).
Allam, J.P. et al. Phl p 5 resorption in human oral mucosa leads to dose-dependent and time-dependent allergen binding by oral mucosal Langerhans cells, attenuates their maturation, and enhances their migratory and TGF-β1 and IL-10-producing properties. J. Allergy Clin. Immunol. 126, 638-645.e1 (2010).
Mascarell, L. et al. Oral macrophage-like cells play a key role in tolerance induction following sublingual immunotherapy of asthmatic mice. Mucosal Immunol. 4, 638–647 (2011).
Pabst, O. & Mowat, A.M. Oral tolerance to food protein. Mucosal Immunol. 5, 232–239 (2012).
Varol, C., Zigmond, E. & Jung, S. Securing the immune tightrope: mononuclear phagocytes in the intestinal lamina propria. Nat. Rev. Immunol. 10, 415–426 (2010).
Merad, M., Sathe, P., Helft, J., Miller, J. & Mortha, A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 31, 563–604 (2013).
Coombes, J.L. et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J. Exp. Med. 204, 1757–1764 (2007).
Sun, C.M. et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 204, 1775–1785 (2007).
Gautier, E.L. et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 13, 1118–1128 (2012).
Tamoutounour, S. et al. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur. J. Immunol. 42, 3150–3166 (2012).
Nagai, Y. et al. Transportation of sublingual antigens across sublingual ductal epithelial cells to the ductal antigen-presenting cells in mice. Clin. Exp. Allergy 45, 677–686 (2015).
Hervouet, C. et al. Antigen-bearing dendritic cells from the sublingual mucosa recirculate to distant systemic lymphoid organs to prime mucosal CD8 T cells. Mucosal Immunol. 7, 280–291 (2014).
Guilliams, M. et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat. Rev. Immunol. 14, 571–578 (2014).
Malissen, B., Tamoutounour, S. & Henri, S. The origins and functions of dendritic cells and macrophages in the skin. Nat. Rev. Immunol. 14, 417–428 (2014).
Tamoutounour, S. et al. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity 39, 925–938 (2013).
Molawi, K. et al. Progressive replacement of embryo-derived cardiac macrophages with age. J. Exp. Med. 211, 2151–2158 (2014).
Henri, S. et al. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J. Exp. Med. 207, 189–206 (2010).
Guilliams, M. et al. Skin-draining lymph nodes contain dermis-derived CD103− dendritic cells that constitutively produce retinoic acid and induce Foxp3+ regulatory T cells. Blood 115, 1958–1968 (2010).
Capucha, T. et al. Distinct murine mucosal Langerhans cell subsets develop from pre-dendritic cells and monocytes. Immunity 43, 369–381 (2015).
Paidassi, H. et al. Preferential expression of integrin αvβ8 promotes generation of regulatory T cells by mouse CD103+ dendritic cells. Gastroenterology 141, 1813–1820 (2011).
Worthington, J.J., Czajkowska, B.I., Melton, A.C. & Travis, M.A. Intestinal dendritic cells specialize to activate transforming growth factor-β and induce Foxp3+ regulatory T cells via integrin αvβ8. Gastroenterology 141, 1802–1812 (2011).
Travis, M.A. & Sheppard, D. TGF-β activation and function in immunity. Annu. Rev. Immunol. 32, 51–82 (2014).
Hadis, U. et al. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity 34, 237–246 (2011).
Tilney, N.L. Patterns of lymphatic drainage in the adult laboratory rat. J. Anat. 109, 369–383 (1971).
Cerovic, V. et al. Intestinal CD103− dendritic cells migrate in lymph and prime effector T cells. Mucosal Immunol. 6, 104–113 (2013).
Cerovic, V., Bain, C.C., Mowat, A.M. & Milling, S.W. Intestinal macrophages and dendritic cells: what's the difference? Trends Immunol. 35, 270–277 (2014).
Mazzini, E., Massimiliano, L., Penna, G. & Rescigno, M. Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1+ macrophages to CD103+ dendritic cells. Immunity 40, 248–261 (2014).
Shi, G.P. et al. Cathepsin S required for normal MHC class II peptide loading and germinal center development. Immunity 10, 197–206 (1999).
Mach, N., Gillessen, S., Wilson, S.B., Sheehan, C., Mihm, M. & Dranoff, G. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 60, 3239–3246 (2000).
Mora, J.R. et al. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature 424, 88–93 (2003).
Schulz, O. et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J. Exp. Med. 206, 3101–3114 (2009).
Varol, C. et al. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity 31, 502–512 (2009).
Hervouet, C. et al. Langerhans cells prime IL-17-producing T cells and dampen genital cytotoxic responses following mucosal immunization. J Immunol. 184, 4842–4851 (2010).
Iwata, M., Hirakiyama, A., Eshima, Y., Kagechika, H., Kato, C. & Song, S.Y. Retinoic acid imprints gut-homing specificity on T cells. Immunity 21, 527–538 (2004).
Schlitzer, A. et al. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity 38, 970–983 (2013).
Barnden, M.J., Allison, J., Heath, W.R. & Carbone, F.R. Defective TCR expression in transgenic mice constructed using cDNA-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 76, 34–40 (1998).
Kawabe, T. et al. Homeostatic proliferation of naive CD4+ T cells in mesenteric lymph nodes generates gut-tropic Th17 cells. J. Immunol. 190, 5788–5798 (2013).
Nagashima, H. et al. The adaptor TRAF5 limits the differentiation of inflammatory CD4+ T cells by antagonizing signaling via the receptor for IL-6. Nat. Immunol. 15, 449–456 (2014).
Bando, K. et al. Resin monomers act as adjuvants in Ni-induced allergic dermatitis in vivo. J. Dent. Res. 93, 1101–1107 (2014).
Acknowledgements
We thank Dr Atsushi Kumanogoh for providing B16-Flt3L cells. We also thank the Biomedical Research Core of Tohoku University Graduate School of Medicine for the use of its equipment. This work was supported by Grants-in-Aid for Scientific Research (B) (24390407 and 15H05011 to S Sugawara), Young Scientists (B) (26861542 to Y Tanaka), and JSPS fellows (267005 to Y Tanaka) from JSPS, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Supplementary information
Rights and permissions
About this article
Cite this article
Tanaka, Y., Nagashima, H., Bando, K. et al. Oral CD103−CD11b+ classical dendritic cells present sublingual antigen and induce Foxp3+ regulatory T cells in draining lymph nodes. Mucosal Immunol 10, 79–90 (2017). https://doi.org/10.1038/mi.2016.46
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2016.46
This article is cited by
-
Transcriptional changes in dendritic cells underlying allergen specific induced tolerance in a mouse model
Scientific Reports (2022)
-
Differential expression of CD11c defines two types of tissue-resident macrophages with different origins in steady-state salivary glands
Scientific Reports (2022)
-
Migratory dendritic cells in skin-draining lymph nodes have nickel-binding capabilities
Scientific Reports (2020)
-
Essential role of submandibular lymph node dendritic cells in protective sublingual immunotherapy against murine allergy
Communications Biology (2020)
-
HIV-1 vaccination by needle-free oral injection induces strong mucosal immunity and protects against SHIV challenge
Nature Communications (2019)