Abstract
We investigated the mucosal distribution and neutralization potency of rhesus recombinant versions of the HIV-specific, broadly neutralizing antibody b12 (RhB12) following intravenous administration to lactating rhesus monkeys. IgG and dimeric IgA (dIgA) administration resulted in high plasma concentrations of broadly neutralizing antibody (bnAb), but the monomeric IgA (mIgA) was rapidly cleared from the systemic compartment. Interestingly, differences in the distribution of the RhB12 isoform were observed between the mucosal compartments. The peak concentration of RhB12 IgG was higher than dIgA in saliva, rectal, and vaginal secretions, but the bnAb concentration in milk was one to two logs higher after dIgA administration than with IgG or mIgA infusion. Neutralization was observed in plasma of all animals, but only those infused with RhB12 dIgA showed moderate levels of virus neutralization in milk. Remarkably, virus-specific secretory IgA was detected in mucosal compartments following dIgA administration. The high milk RhB12 dIgA concentration suggests that passive immunization with dIgA could be more effective than IgG to inhibit virus in breast milk.
Similar content being viewed by others
INTRODUCTION
Despite being responsible for almost half of the 200,000 pediatric HIV-1 infections continuing to occur annually,1 breastfeeding remains the safest way to ensure the survival of HIV-exposed infants in resource-limited areas, because non-breastfed infants have high risk of mortality due to respiratory and diarrheal diseases.2 Breastfed infants born to HIV-1-infected women ingest up to 1 L of virus-containing milk daily, and assuring safe breastfeeding for these infants is a public health priority in areas of high HIV-1 prevalence. Administration of antiretroviral (ARV) drugs to HIV-1-infected mothers or their infants throughout the breastfeeding period can significantly reduce the rate of transmission,3, 4, 5 but the effective implementation of this strategy relies heavily on long-term adherence to the daily drug treatment. However, recent studies reported poor maternal adherence to treatment during the postnatal period,6, 7, 8 suggesting that the elimination of breast milk transmission of HIV-1 will likely require the development of additional preventive interventions.
HIV-1 is present in breast milk as free and cell-associated virus, and levels of both forms of viruses correlate with risk of transmission.9, 10 Although the exact mechanism of HIV-1 transmission via breast milk is still uncertain, results from a study in Botswana have suggested that cell-associated virus accounts for the majority of transmissions until 9 months post partum, whereas free virus is more important later on.11 Importantly, HIV-1 DNA can be detected in the breast milk of ARV-treated patients,12, 13 suggesting that ARV treatment could have a limited impact on the transmission of cell-associated viruses. On the other hand, immune factors such as antibodies can inhibit both cell-free virus and HIV-1-infected cells.
Breast milk is a rich source of antibodies and these antibodies contribute to the protection of infants against common mucosal pathogens during the first months of life. Antibodies in breast milk are either locally produced or they originate from the systemic compartment.14 Although previous studies have demonstrated that HIV-1 Envelope (Env)- specific antibodies are present in the breast milk of infected mothers,15, 16, 17 the contribution of these antibodies to infant protection remains unclear. IgA is the most abundant antibody isotype in the breast milk compartment, but the milk HIV-1 Env-specific antibody response is predominantly IgG. HIV-1 Env-specific IgG levels in milk are two to three folds lower than in plasma, but their magnitude and function correlate with that in plasma, suggesting that the majority of IgG antibodies in milk originate from plasma.15 IgG isolated from breast milk is capable of neutralizing HIV-1 virions with a potency similar to that of plasma, but the overall neutralization potency of breast milk is weak, probably due to the considerably lower levels of Env-specific IgG in milk than in plasma. It was recently reported that the milk neutralizing antibody response does not differ between HIV-1-infected women who did or did not transmit HIV-1 to their infants.17 As only low levels of neutralizing antibodies are detected in milk, induction of robust heterologous neutralizing responses in the milk compartment may be required to achieve effective virus inhibition.
A possible approach to induce effective neutralization is through the passive administration of broadly neutralizing antibodies (bnAbs). Previous studies have established that bnAbs can suppress HIV-1 replication when administered therapeutically.18, 19, 20, 21, 22 The goal of this study was to determine whether virus inhibition could be achieved in breast milk following systemic administration of a bnAb. We used a non-human primate model of lactation23 to study the kinetics of IgG and IgA versions of a bnAb (RhB12) in plasma, milk, and other mucosal compartments following systemic passive immunization. Our results demonstrate differences in the distribution of the RhB12 isoforms, with higher milk bnAb concentration following dimeric IgA (dIgA) compared with IgG or monomeric IgA (mIgA) administration. Importantly, virus neutralization in breast milk was only observed after dIgA infusion. Moreover, low levels of the secretory IgA (sIgA) RhB12 were detected in mucosal compartments following dIgA infusion. The demonstration that systemic administration of a dIgA form of an antibody can yield sIgA in mucosal fluids provides rationale for investigating passive immunotherapy with dIgA as a method to target IgA mucosal delivery and to inhibit pathogens at mucosal sites.
RESULTS
Distribution of RhB12 monoclonal antibodies in blood and milk following systemic passive immunization
We first passively immunized four lactating rhesus monkeys with 5 mg kg−1 of RhB12 IgG (Figure 1). The peak concentration in plasma was observed 1 h after infusion and ranged from 62,548 to 109,070 ng ml−1 (Table 1, Figure 2a). The RhB12 IgG concentration declined over time, but 42 days after infusion, the antibody was still detectable in the plasma at a concentration representing 0.2–3% of the peak antibody levels. The peak concentration in milk was observed 1–3 days post infusion (Figure 2b) and was 2–3 log lower than the peak plasma concentration (range 17–218 ng ml−1). Despite this low peak concentration, 42 days after immunization, RhB12 IgG was still detectable in milk at a concentration representing 42–100% of the peak levels. Thus, systemic administration of 5 mg kg−1 of RhB12 IgG results in low but durable levels of antibodies in breast milk, suggesting a slow clearance of IgG from the milk compartment.
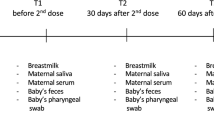
Timeline of RhB12 IgG, monomeric IgA (mIgA), dimeric IgA (dIgA) passive immunization. Lactating rhesus monkeys were immunized intravenously with RhB12 IgG, mIgA or dIgA. Plasma, milk, and mucosal secretions were collected before infusion, and post infusion (1, 6, 24, 72 h, 1 week, and then weekly for 7 weeks).
Passive immunization with RhB12 dimeric IgA (dIgA) yields higher magnitude binding antibodies in milk than RhB12 monomeric IgA (mIgA) or IgG infusion. The time to peak B12 monoclonal antibody (mAb) level in plasma was similar following B12 IgG, mIgA, and dIgA infusion, but the peak plasma mIgA was lower than for IgG or dIgA (a). In milk, mIgA peaked earlier (1–6 h) than IgG (24–72 h) or dIgA (6–24 h). Even though the amount of mIgA infused was three times that of dIgA, the peak level of dIgA in milk was 1- to 2-fold higher and was more durable than that of mIgA (b). Dashed line represents positivity cutoff calculated as three standard deviations above the average concentration measured at time 0 (0.042 days correspond to 1 h and 0.25 days to 6 h).
Because passive immunization with RhB12 IgG only resulted in low milk levels of HIV-1 Env-specific binding antibodies, we next investigated whether higher bnAb concentrations could be achieved with infusion of an IgA form of the antibody. Nine months after the initial infusion, monkeys previously infused with RhB12 IgG were tested for anti-RhB12 antibodies, as antibodies against the bnAb can develop following infusion. All animals showed low levels of plasma reactivity against RhB12 (end titers 10–90, Supplementary Table S1 online), but a comparable low reactivity was also observed in plasma samples collected before infusion, suggesting that animals had pre-existing antibodies that cross-reacted with RhB12. The animals were then immunized intravenously with 15 mg kg−1 of RhB12 mIgA. In addition, three lactating rhesus monkeys were immunized with 5 mg kg−1 of RhB12 dIgA. Similar to IgG, the peak plasma concentrations of RhB12 mIgA and dIgA were observed 1–6 h post infusion (Table 1, Figure 2a). Surprisingly, although the mIgA infusion dose was three times that of IgG, the peak level of RhB12 mIgA was a log lower than that of IgG (range 4,924–11,780 ng ml−1, P=0.03). The plasma levels of mIgA also declined more rapidly than that of IgG and 42 days after infusion, mIgA was no longer detected in plasma. In contrast to mIgA, the peak plasma concentration of dIgA was comparable to that of IgG (range 66,852–87,828 ng ml−1, P=0.57). Two of three monkeys infused with RhB12 dIgA still had low levels of the bnAb in plasma 42 days post infusion at a concentration representing 0.2–0.3% of the peak levels. Thus, RhB12 mIgA appears to be cleared from plasma more rapidly than dIgA or IgG.
The peak RhB12 mIgA concentration in milk was observed 1–6 h after infusion, suggesting a rapid distribution to tissues (Figure 2b). This concentration was comparable to the peak IgG concentration (range 133–386 ng ml−1 vs. 17–218 ng ml−1), but 14 days after immunization, mIgA was no longer detected in breast milk. Interestingly, the peak concentration of dIgA in milk was two logs higher than that of IgG or mIgA (range: 1,929–6,755 ng ml−1, P=0.06 for both). Six weeks after infusion, low levels of RhB12 dIgA were still detectable in the milk of two immunized animals at concentration representing 17 and 21% of the peak levels. Overall, these results indicate differences in the distribution of IgG, mIgA, and dIgA in blood and breast milk following systemic administration.
Distinct pharmacokinetics of IgG, mIgA, and dIgA in plasma and breast milk following systemic administration
A non-compartmental pharmacokinetics (PK) analysis was conducted using the Phoenix WinNolin software (Pharsight Corporation, Mountain View, CA). The median terminal half-life of RhB12 IgG in plasma was 5.26 days, and the median clearance was 9.36 ml kg−1 day−1 (Table 1). These PK parameters of the rhesus recombinant RhB12 are in the same range as that of the human bnAb VRC01 in immunized monkeys (terminal half-life: 4.65 days; clearance: 16.52 ml kg−1 day−1).24 The median terminal half-life of RhB12 dIgA was higher (25.9 days) than that of IgG; yet, IgG appeared to be cleared from plasma at a slower rate than dIgA (median clearance: 9.36 vs. 41 ml kg−1 day−1). The median terminal half-life of RhB12 mIgA in plasma was 4.06 days, but its clearance rate was 200 times faster (2,469 ml kg−1 day−1) than that of RhB12 IgG.
Because the concentration of RhB12 IgG in breast milk did not significantly decrease over time, we were not able to calculate the clearance of the IgG in milk. This parameter could only be computed for one animal passively immunized with RhB12 dIgA (BF38). Because of the rapid drop of mIgA concentration in milk, the clearance could only be calculated for two immunized animals (A6E009 and 01D186). The clearance of RhB12 in milk was 44–109 times higher in animals infused with the mIgA than in the animal infused with the dIgA form of the bnAb (19,216 and 47,654 vs. 436 ml kg−1 day−1). To determine the penetration of the bnAb in the breast milk compartment, the ratio of the area under concentration time curve (AUC 0–42) in breast milk and in plasma was calculated. The median AUC ratio was 0.0017 (range 0.003–0.015) for IgG, 0.037 (range 0.026–0.13) for mIgA, and 0.45 (range 0.072–0.57) for dIgA. The high dIgA milk to plasma AUC ratio suggests a selective transfer of dIgA to the breast milk compartment.
Distribution of RhB12 monoclonal antibodies in other mucosal compartments
We next measured ratio of RhB12 to total IgG/IgA in saliva, vaginal, and rectal fluids (Figure 3). In contrast to breast milk where dIgA was the most abundant RhB12 isoform, in the other mucosal compartments, RhB12 IgG was more abundant. The peak ratio of RhB12 IgG/total IgG was comparable in rectal secretions and saliva (range, rectal: 12–52, saliva: 29–86 ng of RhB12 IgG/μg total IgG, Supplementary Table S3) and was observed 6 h to 3 days after infusion. The peak ratio of RhB12 IgG/total IgG in vaginal secretions was observed 1 h to 3 days post infusion and ranged from 20 to 2,169 ng of RhB12 IgG/μg total IgG. The peak RhB12 mIgA/total IgA ratio in rectal secretions and saliva was observed 1–6 h post infusion and ranged from 1.2 to 6.9 (rectal) and from 0.1 to 4.62 ng RhB12 mIgA/μg total IgA (saliva) whereas in vaginal secretions, the peak ratio was observed 1 h to1 days post infusion (range 0.65–13 ng RhB12 mIgA/μg total IgA). Finally, the peak RhB12 dIgA/total IgA ratio was observed 6 h to 3 days after infusion in rectal, saliva, and vaginal secretions (rectal: 2.66–18; saliva: 4.54–7.15 ng, vaginal:13.1–70 μg RhB12 IgA/μg total IgA). These results indicate differences in the distribution of the bnAb isoforms to various mucosal compartments following systemic administration. Thus, different isoforms of systemically-administered bnAb may be required to optimally protect from distinct routes of mucosal HIV-1 exposure.
Differences in the kinetics of B12 monoclonal antibody (mAb) in mucosal compartments following RhB12 IgG, monomeric IgA (mIgA), dimeric IgA (dIgA) systemic infusion. The ratio of HIV-1 Env-specific to total IgG/IgA antibody was higher for IgG than mIgA/dIgA in rectal (a), saliva (b), and vaginal (c) secretions, whereas the ratio of Env-specific to total IgG/IgA was higher for dIgA in milk (d). Lines represent median. 0.042 days correspond to 1 h and 0.25 days correspond to 6 h.
Detection of sIgA in mucosal compartments following dIgA passive immunization
We then determined whether HIV-1 Env-specific sIgA could be detected in mucosal compartments following systemic administration of RhB12 dIgA. With the exception of saliva from one animal (BB 25), RhB12 sIgA was detected at least at one time point in all the mucosal compartments (Figure 4). When detected, the concentration of RhB12 sIgA was often similar or higher than that of dIgA in saliva, rectal, and vaginal secretions, indicating that the sIgA represented a major fraction of RhB12 dIgA in these compartments. Importantly, while the proportion of RhB12 dIgA that was sIgA was lower in breast milk than in other mucosal compartments (Figure 4), the total concentration of RhB12 sIgA was higher in breast milk. These results provide the proof of principle that dIgA administered intravenously can acquire the secretory component during transport to mucosal sites.
Detection of RhB12 secretory IgA (sIgA) in mucosal compartments following systemic RhB12 dimeric IgA (dIgA) passive immunization. At the peak RhB12 level, Env-specific sIgA was detected in all mucosal compartments: rectal (a), saliva (b), vagina (c), milk (d), with lower sIgA (gray bar)/dIgA (black bar) in milk than in other mucosal compartments.
Neutralization potency of systemically-administered RhB12 mAb isoforms in plasma and milk
The neutralization potency of the RhB12 isoforms was assessed in TZM-bl cells against two tier 1 (HIV MW965 and HIV BAL) and five tier 2 viruses (HIV RHPA 4252.7, HIV WITO, HIV CH40, HIV CH58, and HIV AC10.0.29, Supplementary Table 2). The tier 1 viruses were neutralized with a comparable potency by the three antibodies. At the highest tested concentration (25 μg ml−1), one of the tier 2 viruses (HIV WITO) was not neutralized by either RhB12 mIgA or dIgA, despite moderate neutralization of this virus by RhB12 IgG (IC50: 11.03 μg ml−1). Although the IC50 of the mIgA was slightly higher than that of the IgG or dIgA form of RhB12 against several variants, overall, there was no major difference in the neutralization potency of the RhB12 isoforms.
Next, the plasma neutralizing potency of the passively immunized animals was assessed in TZM-bl cells. The plasma of all passively-infused monkeys was able to neutralize the tier 1 virus HIV MW965 and the peak neutralization titer was observed 1–6 h after immunization (Figure 5). Yet, the peak neutralization titer of mIgA was significantly lower than that of IgG (ID50 range: 246–553 vs. 1,875–3,128; P=0.03, Table 2), and by 7 days after infusion, neutralization was no longer detected in the plasma following RhB12 mIgA administration. In contrast, 42 days after RhB12 IgG infusion, neutralization could still be detected in plasma. The peak neutralization titer following RhB12 dIgA infusion was comparable to that of IgG (range: 992–2,283; P=0.22) but 21 days after infusion, neutralization was no longer detected in the plasma. These data are in accordance with a more rapid clearance of RhB12 dIgA from plasma as compared with RhB12 IgG.
Tier 1 HIV-1 neutralization by plasma of RhB12 passively immunized monkeys. The peak neutralization activity achieved in plasma was higher following RhB12 IgG infusion (a) than b12 monomeric IgA (mIgA) (b). Plasma neutralization following RhB12 dimeric IgA (dIgA) infusion is high at peak but short-lived (c). 0.042 days correspond to 1 h and 0.25 days correspond to 6 h.
We next determined the neutralization potency of the plasma from the immunized animals against a panel of tier 1 and tier 2 HIV-1 viruses (Supplementary Table 4). Plasma from all the infused animals neutralized HIV BAL.26 (tier 1), and HIV RHPA 4252.7 (tier 2) whereas HIV WITO and HIV AC10.0.29 were resistant to neutralization, in accordance with the relatively weak neutralization of these viruses by the infused mAbs (Supplementary Table 2).
Detection of breast milk virus neutralization following systemic dIgA administration
Finally, we assessed the ability of breast milk of immunized animals to neutralize the tier 1 virus MW965 (Table 2). As breast milk can mediate non-specific virus neutralization,25 a sample was considered to mediate neutralization if the post-immunization titer was three times higher than the pre-immune titer and three times higher than the neutralization titer of a control virus (SVA. MLV). On the basis of these criteria, low levels of neutralization were observed in one animal post-RhB12 IgG immunization and in two animals after RhB12 mIgA immunization (Table 2). In all these instances, no detectable levels of neutralization were expected based on the low levels of bnAb in milk, suggesting non-specific activity. In contrast, consistent with their higher levels of RhB12 dIgA in milk, moderate levels of neutralizing activity were observed in the breast milk of animals immunized with RhB12 dIgA (peak neutralization ID50 range 212–757). There was a strong correlation between the expected neutralization titers calculated based on the RhB12 concentration in milk and the neutralization titers measured in these animals (r=0.85, P=0.001, Supplementary Figure S3). Thus, systemic administration of a dIgA bnAb can result in efficient mAb transfer to mucosal compartments and to virus neutralization activity in a mucosal compartment important for protecting infants against HIV-1 acquisition.
DISCUSSION
Although ARV therapy significantly reduces cell-free virus load in milk, its impact on cell-associated viruses is moderate.26, 27 Nevertheless, whether the inability of maternal ARV therapy to completely abrogate postnatal infant infection is due to its limited ability to block transmission of HIV-1-infected cells remains unknown. If indeed cell-associated viruses contribute to breast milk transmission, even in the setting of perfect maternal ARV coverage and adherence, then ARV alone will not be sufficient to eliminate postnatal infant HIV-1 infections.
Passive immunization of HIV-1-infected women with bnAbs could constitute an effective strategy to reduce postnatal transmission of HIV-1. This strategy may be attractive for several reasons: (i) antibodies can neutralize virus as well as recruit innate effector cells that can eliminate infected cells; (ii) the long half-life of antibodies may circumvent adherence issues during the postpartum period; and (iii) immune complexes of passively administered antibodies may enhance the quality of the HIV-1-specific immune response in infected mothers.28 Recent studies in animal models have demonstrated that administration of bnAbs to infected animals can significantly reduce plasma viremia.19, 29 Moreover, it was recently established that passive immunization of HIV-1-infected individuals with a single bnAb (3BNC117) is well tolerated and can significantly reduce plasma viremia for up to 28 days.30 Interestingly, administration of the V3 glycan-directed bnAb PGT121 to rhesus monkeys resulted not only in a decrease in plasma virus load, but also to a significant reduction in cell-associated virus levels in peripheral blood, lymph node, and gastrointestinal mucosa with no development of resistant viral strains.29 Although our study demonstrates that systemic passive immunization can result in bnAb levels in breast milk capable of mediating HIV-1 virus neutralization in vitro, whether passive immunization with a bnAb can impact breast milk viral load is still unknown. Future studies should therefore seek to determine whether the presence of bnAbs in milk results in a reduction of cell-free and/or cell-associated virus load.
Our results indicate profound differences in the kinetics and distribution of RhB12 isoforms following systemic administration. Notably, we observed that the clearance of IgG in plasma was slower than that of dIgA; yet, mIgA was rapidly cleared from the systemic compartment (Table 1). In addition, mIgA was rapidly cleared from the breast milk compartment whereas RhB12 IgG and dIgA were still detectable several weeks after infusion. Surprisingly, administration of a similar dose of RhB12 IgG and dIgA resulted in significantly higher concentrations of dIgA in breast milk, whereas IgG was the most abundant isoform in saliva, as well as in rectal and vaginal secretions. These differences may be related to differences in the transport of IgG and dIgA to mucosal compartments. IgG is transported to mucosal sites either by passive diffusion or through interaction with the Fc neonatal receptor (FcRn). FcRn binds to the Fc portion of IgG at acidic pH and protects IgG from degradation in the endosome. IgG is then released in the extracellular space as binding is virtually absent at physiological pH. Thus, FcRn is critical to the bilateral transport of IgG across epithelial barriers and to IgG homeostasis. It was recently reported that a mutation in IgG Fc region (M428L/N434S) increases the serum half-life (from 4.65 to 11.80 days) and the mucosal localization of the bnAb VRC01 IgG.24 Thus, it is possible that FcRn contributed to the RhB12 IgG persistence in plasma and mucosal fluids that we observed. Some studies have suggested that Fc glycosylation could impact the clearance of IgG, but these results were not observed in other studies (reviewed in ref. 31) and little is known about the role of glycosylation in IgA clearance. We did not compare the glycoprofile of the RhB12 IgG and IgA mAbs, yet they were produced in the same cell line. In addition, rhesus macaques have only one IgA subclass which is the ortholog of human IgA2. Thus, rhesus IgA lacks the heavily glycosylated hinge region and has no O-linked glycosylation sites. It is therefore unlikely that Fc glycosylation contributes to explain the different kinetics observed. In future studies, the PK findings from this study can be used to develop paired models between plasma and breast milk/tissues to estimate the passive immunization dosing regimens required to achieve efficacious exposure in mucosal tissues.
MIgA is transported to mucosal sites by passive diffusion, whereas dIgA is transported either by passive diffusion or through interaction with the polyimmunoglobulin receptor (pIgR) expressed on epithelial cells. Upon binding to dIgA at the baso-lateral surface of the epithelial cell, pIgR is endocytosed then transported to the apical site where IgA is released as two mIgA molecules linked by the J chain peptide and the extracellular ligand binding portion of pIgR (secretory component). It has been postulated that sIgA could mediate unique effector functions such as aggregation of virions and inhibition of their movement through mucous and epithelial layers,32 but whether these functions are important for mucosal virus clearance is unknown. SIgA was detected in all mucosal compartments following dIgA systemic administration (Figure 4). The presence of the secretory component, which confers resistance to degradation by non-specific microbial and digestive proteases, may contribute to explain the persistence of RhB12 dIgA in mucosal secretions as compared with mIgA. Importantly, in the moderately effective RV144 vaccine trial, high levels of HIV-1 Env-specific IgA antibodies in plasma were associated with increased risk of HIV-1 acquisition among vaccines.33 IgA in serum is mainly monomeric, whereas in mucosal fluid, polymeric IgA is predominant. As mucosal IgA responses were not measured in RV144 samples, it is unknown whether vaccine-induced mucosal IgA responses are associated with higher HIV-1 acquisition risk. Our results showing differences in the kinetics and the distribution of dIgA and mIgA to mucosal compartments highlight the importance of specifically measuring antibody levels, isoform, and function in mucosal samples.
Although most studies of passive immunization have focused on administration of IgG bnAbs, a few recent studies indicated that IgA bnAbs could also prevent mucosal HIV-1 acquisition. In a humanized mice study, passive immunization with polymeric IgA b12 protected animals from vaginal HIV-1 exposure more effectively than the mIgA or IgG1 b12.34 Similarly, local administration of a neutralizing dIgA antibody was shown to provide better protection than IgG against exposure to a simian human immunodeficiency virus in rectally-challenged rhesus monkeys.35 Interestingly, it was reported that systemic passive immunization with polymeric IgA was 10 times more effective at reducing influenza virus shedding in murine nasopharynx than IgG infusion.36 Moreover, a recent study indicated that intranasal infusion of mice with sIgA isolated from human colostrum can protect from Mycobacterium tuberculosis infection.37 Local administration of IgA has been evaluated in several human and animal models studies as a strategy to fight against respiratory and gastro-intestinal tract infections such as periodontal disease, flu, or cholera (reviewed in ref. 38). Furthermore, oral passive immunization with sIgA has been proposed as a potential strategy to treat immunodeficient patients with gastro-intestinal infection manifestations.39 The high bnAb magnitude achieved in breast milk following the administration of RhB12 dIgA and the persistence of the bnAb in breast milk suggest that systemic dIgA passive immunization could also have important clinical benefits for targeting antibody to mucosal compartments. A potential obstacle to systemic administration of IgA is the risk of development of anaphylactic reactions in patients with IgA deficiency. Fortunately, IgA deficiency is rare,40 and the majority of patients with anti-IgA antibodies do not develop anaphylactic reactions following administration of blood products containing traces of IgA.41 Nevertheless, IgA-based passive immunotherapy will require prior testing of patients for IgA deficiency and anti-IgA antibodies. Finally, defining the bnAb or bnAb combinations that are more likely to impact both cell-free and cell-associated viruses in breast milk is critical for the development of maternal passive immunization strategies to eliminate postnatal HIV-1 transmission.
METHODS
Generation and production of rhesus recombinant b12 antibodies. Immunoglobulin heavy and light chain variable regions of the bnAb b12 (ref. 42) were synthesized and subcloned into expression vectors containing the Ig constant regions of rhesus kappa light chain (GenBank: FJ795854.1) and rhesus IgG1 heavy chain (GenBank: AY292502.1) or rhesus IgA heavy chain43 (GenBank: AY039248.1) sequences. Recombinant heavy and light chain vectors were packaged in retroviral vectors. Complete immunoglobulins were expressed by co-infection of CHO cells with heavy and light chain retroviral vectors using the GPEx expression technology (Catalent Pharma Solutions, Somerset, NJ). Recombinant b12-rhesus IgG was expressed using recombinant IgG1 heavy chain and recombinant light chain vectors. Monomeric b12-rhesus IgA was expressed using recombinant IgA heavy chain and recombinant light chain vectors. Dimeric b12-rhesus IgA was expressed by re-transduction of the IgA-expressing CHO cells with a vector containing rhesus immunoglobulin J polypeptide linker (J-chain) sequence (Genbank: NM_001260886.1). Pools of transduced cells were grown in serum-free medium. Secreted IgG antibody was purified by protein A affinity chromatography (MabSelect Protein A Affinity Media, GE). Secreted IgA antibodies were purified using other affinity chromatographic methods (CaptureSelect IgA Affinity Matrix, Life Technologies, Carlsbad, CA or Fabsorbant F1P HC, ProMetic Bioscience, Laval, Québec, Canada). Purified antibodies were placed in phosphate buffer pH 7, initially characterized by SDS-PAGE, quantified by absorbance at A280, and confirmed to contain endotoxin levels <1 EU mg−1. Evaluation for Ig monomers, dimmers, and aggregates was performed by gel filtration. A total of 300 μg of antibody at 1.0 mg ml−1 was resolved using a Superdex 200 10/300 GL column and an Äkta explorer (GE Healthcare, Little Chalfont, Buckinghamshire, UK). A flow rate of 0.70 ml min−1 was used, and A280 absorbance was measured for 25 min (Supplementary Figure S1). The ability of the mAbs to bind HIV Env was confirmed by ELISA (Supplementary Figure S2).
Animals and immunization schedule. Lactation was induced in seven female Indian rhesus monkeys by depot medroxyprogesterone and estradiol injections followed by oral administration a dopamine antagonist, as described.23 RhB12 IgG was administered intravenously over 5 min to four hormone-induced, lactating animals at a dose of 5 mg kg−1 (Figure 1). RhB12 mIgA was administered to the same animals 9 months after the IgG infusion at a dose of 15 mg kg−1. Before infusion, plasma samples were tested for reactivity against RhB12. RhB12 dIgA was administered at a dose of 5 mg kg−1 to three lactating rhesus monkeys. Milk and blood were collected 1, 6, 24, and 72 h post infusion, then weekly for 8 weeks. In addition, saliva, vaginal, and rectal secretions were collected using pre-moistened weck-cel surgical sponges (Medtronic, Jacksonville, FL).44 The animals were maintained according to the “Guide for the Care and Use of Laboratory Animals.”
Processing of mucosal samples. Mucosal secretions were eluted from weck-cel sponges using a reported protocol44 with slight modifications. Briefly, the elution buffer was prepared by adding 50 μl of 100 × protease inhibitor cocktail and 250 μl of Igepal (Sigma, St Louis, MO) to 4.7 ml of phosphate buffer saline containing 0.25% of bovine serum albumin (Sigma). Sponges were thawed at room temperature then transferred tip down to the upper chamber of a spinX column (Corning Life Sciences, Lowell, MA), then 50 μl of ice-cold elution buffer was added. The buffer was allowed to diffuse for 15 min followed by a centrifugation of the tubes at 20,000 g at 4 °C for 10 min. The procedure was repeated twice, and then the eluted fractions were concentrated.
Measurement of HIV-1 Env and total IgA/IgG by ELISA. 384-well ELISA plates (Corning Life Sciences) were coated overnight at 4 °C with either con6 gp120 or goat anti-Monkey IgG/IgA (Rockland, Gilbertsville, PA), then blocked with superblock buffer (phosphate-buffered saline containing 4% whey, 15% normal goat serum, and 0.5% Tween-20) before addition of diluted samples. IgG antibodies were detected using a HRP-conjugated goat anti-monkey IgG (Rockland). IgA antibodies were detected using α-rhesus IgA 10F12 Biotin (Nonhuman Primate Reagent Resource), followed by HRP-conjugated streptavidin (ThermoScientific, Rockford, IL). SIgA was detected using a mouse anti-α-secretory chain IgG (Ab SC 9H7 CL3), which cross-reacts with rhesus and human secretory component (DHVI), followed by a HRP-conjugated, polyclonal anti-mouse IgG (Promega, Madison, WI). The SureBlue Reserve tetramethylbenzidine peroxidase substrate (KPL, Gaithersburg, MD) was added to plates, and plates were read at 450 nm immediately after addition of the stop solution. RhB12 IgG, mIgA dIgA, sIgA, and Macaca mulatta purified IgA or IgG (Nonhuman Primate Reagent Resource) were used as standards. The RhB12 sIgA standard was prepared by complexing RhB12 dIgA with Rhesus SC followed by an overnight incubation, resulting in a 1:1 molar ratio of dIgA:SC. Antibody concentrations were calculated relative to standard using a 5-parameter fit curve (WorkOut 2.5; PerkinElmer, Waltham, MA). To account for non-specific background binding of samples to the HIV-1 Envelope, the positivity cutoff was defined as the median+3 standard deviation of the calculated pre-infusion concentrations.
Neutralization assays. Neutralization was measured in TZM-bl cells by reduction in luciferase reporter gene expression after a single round of infection, as described.45 Because of their non-specific neutralization activity, breast milk samples were tested against HIV virus and the murine leukemia virus SVA.MLV46 (negative control). The 50% inhibitory dose (ID50) titer was calculated as the plasma or milk dilution that caused a 50% reduction in relative luminescence units compared with the virus control wells after subtraction of cell control relative luminescence units. As unfractionated breast milk has been shown to be toxic to TZM-bl cells, breast milk samples were delipidized by high speed centrifugation at 4 °C followed by filtration before use in neutralization assays as previously described.15
Non-compartmental PK analysis. Individual subject non-compartmental pharmacokinetic analysis was performed using the Phoenix WinNolin software. Plasma and breast milk were analyzed separately. The analysis assumed an IV bolus administration in plasma and an extracellular distribution in breast milk. Only time points with concentration above the positivity cutoff were included in the analysis. The PK parameters calculated with the observed data included the maximal concentration (Cmax), the time to the maximal concentration (Tmax), the AUC of 0–42 days, and the terminal elimination half-life using the terminal slope of the concentration vs. time curve. The terminal slope was determined from log-linear portion of the curve using at least three data points, and the terminal half-life was determined as  . The area under the curve was calculated with the trapezoidal method.
. The area under the curve was calculated with the trapezoidal method.
Statistical analysis. Statistical analysis was conducted using GraphPad prism 6 (San Diego, CA). The Mann–Whitney U-test was used to compare RhB12 isoforms levels in plasma and breast milk. The correlation between expected and observed neutralization titers was assessed using the Pearson correlation coefficient.
References
UNAIDS 2014 Progress report on the global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive.
Bork, K.A. et al. Morbidity in relation to feeding mode in African HIV-exposed, uninfected infants during the first 6 mo of life: the Kesho Bora study. Am. J. Clin. Nutr. 100, 1559–1568 (2014).
Fowler, M.G. et al. Efficacy and safety of an extended nevirapine regimen in infants of breastfeeding mothers with HIV-1 infection for prevention of HIV-1 transmission (HPTN 046): 18-month results of a randomized, double-blind, placebo-controlled trial. J. Acquir. Immune Defic. Syndr. 65, 366–374 (2014).
Kesho Bora Study Group de Vincenzi, I. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect. Dis. 11, 171–180 (2011).
Thomas, T.K. et al. Triple-antiretroviral prophylaxis to prevent mother-to-child HIV transmission through breastfeeding—the Kisumu Breastfeeding Study, Kenya: a clinical trial. PLoS Med. 8, e1001015 (2011).
Phillips, T., Thebus, E., Bekker, L.G., McIntyre, J., Abrams, E.J. & Myer, L. Disengagement of HIV-positive pregnant and postpartum women from antiretroviral therapy services: a cohort study. J. Int. AIDS Soc. 17, 19242 (2014).
Ngoma, M.S. et al. Efficacy of WHO recommendation for continued breastfeeding and maternal cART for prevention of perinatal and postnatal HIV transmission in Zambia. J Int AIDS Soc 18, 19352 (2015).
Ngarina, M. et al. Virologic and immunologic failure, drug resistance and mortality during the first 24 months postpartum among HIV-infected women initiated on antiretroviral therapy for life in the Mitra plus Study, Dar es Salaam, Tanzania. BMC Infect. Dis. 15, 175 (2015).
Rousseau, C.M. et al. Association of levels of HIV-1-infected breast milk cells and risk of mother-to-child transmission. J. Infect. Dis. 190, 1880–1888 (2004).
Semba, R.D. et al. Human immunodeficiency virus load in breast milk, mastitis, and mother-to-child transmission of human immunodeficiency virus type 1. J. Infect. Dis. 180, 93–98 (1999).
Koulinska, I.N. et al. Transmission of cell-free and cell-associated HIV-1 through breast-feeding. J. Acquir. Immune Defic. Syndr. 41, 93–99 (2006).
Slyker, J.A. et al. Incidence and correlates of HIV-1 RNA detection in the breast milk of women receiving HAART for the prevention of HIV-1 transmission. PLoS One 7, e29777 (2012).
Lehman, D.A. et al. HIV-1 persists in breast milk cells despite antiretroviral treatment to prevent mother-to-child transmission. AIDS 22, 1475–1485 (2008).
Larson, B.L., Heary, H.L. Jr. & Devery, J.E. Immunoglobulin production and transport by the mammary gland. J. Dairy Sci. 63, 665–671 (1980).
Fouda, G.G. et al. HIV-specific functional antibody responses in breast milk mirror those in plasma and are primarily mediated by IgG antibodies. J. Virol. 85, 9555–9567 (2011).
Van de Perre, P., Hitimana, D.G. & Lepage, P. Human immunodeficiency virus antibodies of IgG, IgA, and IgM subclasses in milk of seropositive mothers. J. Pediatr. 113, 1039–1041 (1988).
Mabuka, J., Nduati, R., Odem-Davis, K., Peterson, D. & Overbaugh, J. HIV-specific antibodies capable of ADCC are common in breastmilk and are associated with reduced risk of transmission in women with high viral loads. PLoS Pathog. 8, e1002739 (2012).
Stoddart, C.A. et al. Efficacy of broadly neutralizing monoclonal antibody PG16 in HIV-infected humanized mice. Virology 462–463, 115–125 (2014).
Klein, F et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature 492, 118–122 (2012).
Horwitz, J.A. et al. HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proc. Natl. Acad. Sci. USA 110, 16538–16543 (2013).
Diskin, R. et al. Restricting HIV-1 pathways for escape using rationally designed anti-HIV-1 antibodies. J. Exp. Med. 210, 1235–1249 (2013).
Halper-Stromberg, A. et al. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell 158, 989–999 (2014).
Permar, S.R. et al. Potent simian immunodeficiency virus-specific cellular immune responses in the breast milk of simian immunodeficiency virus-infected, lactating rhesus monkeys. J. Immunol. 181, 3643–3650 (2008).
Ko, S.Y. et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature 514, 642–645 (2014).
Fouda, G.G. et al. Tenascin-C is an innate broad-spectrum, HIV-1-neutralizing protein in breast milk. Proc. Natl. Acad. Sci. USA 110, 18220–18225 (2013).
Milligan, C. & Overbaugh, J. The role of cell-associated virus in mother-to-child HIV transmission. J. Infect. Dis. 210, S631–S640 (2014).
Van de Perre, P. et al. HIV-1 reservoirs in breast milk and challenges to elimination of breast-feeding transmission of HIV-1. Sci. Transl. Med. 4, 143sr143 (2012).
Whaley, K.J. & Mayer, K.H. Strategies for preventing mucosal cell-associated HIV transmission. J Infect Dis 210, S674–S680 (2014).
Barouch, D.H. et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 503, 224–228 (2013).
Caskey, M et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 522, 487–491 (2015).
Correia, I.R. Stability of IgG isotypes in serum. MAbs 2, 221–232 (2010).
Hladik, F. & Hope, T.J. HIV infection of the genital mucosa in women. Curr. HIV/AIDS Rep. 6, 20–28 (2009).
Haynes, B.F. et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 366, 1275–1286 (2012).
Hur, E.M. et al. Inhibitory effect of HIV-specific neutralizing IgA on mucosal transmission of HIV in humanized mice. Blood 120, 4571–4582 (2012).
Watkins, J.D. et al. Anti-HIV IgA isotypes: differential virion capture and inhibition of transcytosis are linked to prevention of mucosal R5 SHIV transmission. AIDS 27, F13–F20 (2013).
Renegar, K.B., Small, P.A. Jr., Boykins, L.G. & Wright, P.F. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J. Immunol. 173, 1978–1986 (2004).
Alvarez, N. et al. Passive administration of purified secretory IgA from human colostrum induces protection against Mycobacterium tuberculosis in a murine model of progressive pulmonary infection. BMC Immunol. 14, S3 (2013).
Corthesy, B. Recombinant secretory immunoglobulin A in passive immunotherapy: linking immunology and biotechnology. Curr. Pharm. Biotechnol. 4, 51–67 (2003).
Carbonare, C.B., Carbonare, S.B. & Carneiro-Sampaio, M.M. Secretory immunoglobulin A obtained from pooled human colostrum and milk for oral passive immunization. Pediatr. Allergy Immunol. 16, 574–581 (2005).
Palmer, D.S. et al. Screening of Canadian Blood Services donors for severe immunoglobulin A deficiency. Transfusion 50, 1524–1531 (2010).
Rachid, R., Castells, M., Cunningham-Rundles, C. & Bonilla, F.A. Association of anti-IgA antibodies with adverse reactions to gamma-globulin infusion. J. Allergy Clin. Immunol. 128, 228–230.e221 (2011).
Burton, D.R., Barbas, C.F. 3rd, Persson, M.A., Koenig, S., Chanock, R.M. & Lerner, R.A. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc. Natl. Acad. Sci. USA 88, 10134–10137 (1991).
Rogers, K.A., Jayashankar, L., Scinicariello, F. & Attanasio, R. Nonhuman primate IgA: genetic heterogeneity and interactions with CD89. J. Immunol. 180, 4816–4824 (2008).
Kozlowski, P.A., Lynch, R.M., Patterson, R.R., Cu-Uvin, S., Flanigan, T.P. & Neutra, M.R. Modified wick method using Weck-Cel sponges for collection of human rectal secretions and analysis of mucosal HIV antibody. J. Acquir. Immune Defic. Syndr. 24, 297–309 (2000).
Montefiori, D.C. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol. Biol. 485, 395–405 (2009).
Seaman, M.S. et al. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J. Virol. 84, 1439–1452 (2010).
Acknowledgements
This work was supported in part by a Collaboration for AIDS Vaccine Discovery (CAVD) grant from the Bill & Melinda Gates Foundation (OPP1040758), by a K08 grant from the National Institute of Health grant (AI087992 to SP) and by a grant from the National Center For Advancing Translational Sciences, NIH (KL2TR001115 to GF). Research reported in this manuscript was supported by a grant from the National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS for the Center for HIV/AIDS Vaccine Immunology (CHAVI; U19-AI067854). The sponsor had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Antibodies used in these studies were produced by the NIH Nonhuman Primate Reagent Resource funded by NIH grant OD010976 and NIAID contract HHSN272200130031C.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Supplementary information
Rights and permissions
About this article
Cite this article
Fouda, G., Eudailey, J., Kunz, E. et al. Systemic administration of an HIV-1 broadly neutralizing dimeric IgA yields mucosal secretory IgA and virus neutralization. Mucosal Immunol 10, 228–237 (2017). https://doi.org/10.1038/mi.2016.32
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2016.32