Abstract
Elevated levels of interleukin-18 (IL-18) are found in many chronic inflammatory disorders, including inflammatory bowel disease (IBD), and polymorphisms in the IL18R1–IL18RAP locus are associated with IBD susceptibility. IL-18 is an IL-1 family cytokine that has been proposed to promote barrier function in the intestine, but the effects of IL-18 on intestinal CD4+ T cells are poorly understood. Here we demonstrate that IL-18R1 expression is enhanced on both effector and regulatory CD4+ T cells in the intestinal lamina propria, with T helper type 17 (Th17) cells exhibiting particularly high levels. We further show that, during steady state, intestinal epithelial cells constitutively secrete IL-18 that acts directly on IL-18R1-expressing CD4+ T cells to limit colonic Th17 cell differentiation, in part by antagonizing IL-1R1 signaling. In addition, although IL-18R1 is not required for colonic Foxp3+ regulatory T (Treg) cell differentiation, we found that IL-18R1 signaling was critical for Foxp3+ Treg cell–mediated control of intestinal inflammation, where it promoted the expression of key Treg effector molecules. Thus IL-18 is a key epithelial-derived cytokine that differentially regulates distinct subsets of intestinal CD4+ T cells during both homeostatic and inflammatory conditions, a finding with potential implications for treatment of chronic inflammatory disorders.
Similar content being viewed by others
INTRODUCTION
Intestinal immune homeostasis is maintained through a constant molecular dialogue between commensal microbiota, intestinal tissue cells, and the mucosal immune system.1 Breakdown of this mutualistic relationship results in chronic pathologies of the gastrointestinal tract, including inflammatory bowel disease (IBD).2 T helper type 17 (Th17) cells, dependent on the transcription factor retinoic acid-related orphan receptor-γt (Rorγt), represent a distinct interleukin (IL)-17A-producing CD4+ T-cell subset that contribute both to host defense from pathogens and to tissue pathologies in a number of inflammatory diseases and experimental models, including colitis.3 Conversely, Foxp3+ regulatory T (Treg) cells prevent systemic and tissue-specific autoimmunity and are crucial for intestinal immune homeostasis.4 In addition to induction under inflammatory conditions, Th17 cells are present within the gastrointestinal tract under homeostatic conditions. Intestinal Th17 cell differentiation occurs upon colonization by commensal microbes and is dependent upon IL-1R1 signaling on CD4+ T cells.5, 6, 7 IL-1 family cytokines are key co-regulators of CD4+ T-cell fate, and the role of IL-1β in Th17 cell differentiation is mirrored by the contribution of IL-33 and IL-18 to Th2 and Th1 cell subsets, respectively.8 Although IL-18 is not essential for Th1 cell differentiation, under inflammatory conditions, IL-12 signaling promotes IL-18R1 expression on differentiating Th1 cells, whereupon IL-18 stimulation acts to enhance interferon (IFN)-γ production.9, 10, 11
Genome-wide association studies have revealed a number of polymorphisms associated with disease susceptibility, including association of mutations within the IL18R1–IL18RAP locus with both adult and severe early-onset IBD.12, 13, 14 Furthermore, intestinal biopsies from IBD patients produced increased concentrations of IL-18, and exacerbated Th1 cell responses are found in patients with IBD.15, 16 Murine models of CD4+ T-cell-mediated colitis have also attributed a pathogenic role to IL-18 in the intestine.17 Conversely, recent studies in mice lacking key inflammasome components that regulate the processing and secretion of IL-18 have proposed a tissue-protective role for IL-18 following injury to the intestinal epithelium.18, 19 Therefore, the role of IL-18 in intestinal immune regulation, as well as the key cellular sources of this cytokine in the gut, remain unclear.20 Here we demonstrate that intestinal epithelial cells (IECs) regulate colonic CD4+ T-cell homeostasis through production of IL-18. Under homeostatic conditions, IL-18R1 signaling limited colonic Th17 cell differentiation, whereas during inflammation, Foxp3+ Treg cell expression of IL-18R1 was critical for prevention of experimental colitis.
RESULTS
IL-18R1+ CD4+ T cells are enriched in the colonic lamina propria
A diverse range of effector and regulatory CD4+ T cells populate the colonic lamina propria; however, the role of IL-18R signaling on distinct CD4+ T-cell subsets within the intestine remains unknown. To determine whether IL-18/IL-18R interactions might influence colonic CD4+ T cells, we first investigated the expression of IL-18R components, IL-18R1 and IL-18RaP, on CD4+ T-cell subsets polarized in vitro. We detected high Il18r1 and Il18rap expression on Th1, Th17, and induced Treg cells compared with naive CD4+ T cells or those cultured under Th0- or Th2-polarizing conditions (Figure 1a). Efficient polarization was confirmed by expression of subset-restricted genes (see Supplementary Figure S1 online). To confirm these observations in vivo, we performed flow cytometric analysis of IL-18R1 expression by CD4+ T cells present under homeostatic conditions within the colonic lamina propria. In accordance with our in vitro observations, IL-18R1 expression by naive (CD62L+ CD44-) CD4+ T cells was low relative to effector/memory (CD44+ CD62L−) CD4+ T cells, both in the spleen and colon (Figure 1b). Furthermore, although IL-18R1 expression was evident on colonic Th1, Th17, and Foxp3+ Treg cells (Figure 1c), the proportions of IL-18R1+ cells varied among colonic CD4+ T-cell subsets, with IL-18R1 expressed almost ubiquitously by Th17 cells (91.7±2.4%), on the majority of Th1 cells (70.5±2.6%), and on a significant proportion of Foxp3+ Treg cells (40.7±2.7%; Figure 1d). Moreover, while IL-18R1 expression was also detectable on CD4+ T-cell subsets from the spleen and mesenteric lymph node (MLN), the frequency of IL-18R1+ CD4+ T cells was significantly lower on all CD4+ T-cell subsets isolated from peripheral lymphoid tissues when compared with their colonic counterparts (Figure 1d). These data demonstrate that IL-18R1+ CD4+ T cells are enriched within the colonic lamina propria, particularly among Th17 cells, and raised the possibility that IL-18 may have an important regulatory role on colonic CD4+ cell populations.
IL-18R1+ CD4+ T cells are enriched within the colonic lamina propria. (a) Naive CD4+ T cells were polarized to T helper type 0 (Th0), Th1, Th2, Th17, and induced regulatory T (Treg) cell lineages and gene expression assayed by quantitative reverse transcriptase–PCR. (b) Representative fluorescence-activated cell-sorted (FACS) histograms of interleukin (IL)-18R1 expression by splenic and colonic CD62L+ CD44− and CD44+ CD62L− CD4+ T cells from wild-type (WT) mice. (c) Representative FACS plots of IL-18R1 expression by colonic Th1, Th17, and Foxp3+ Treg cells from WT mice. (d) Frequencies of IL-18R1+ Th1, Th17, and Foxp3+ Treg cells from the spleen, mesenteric lymph node (MLN), and colon of WT mice. Data are shown as mean±s.e.m. and represents results from two to three independent experiments with consistent results. Each dot represents an individual mouse with n=4–5 mice/group. IFN, interferon.
Canonical IL-18R1 signaling limits colonic Th17 cell differentiation
To determine the impact of IL-18 on colonic CD4+ T cells, we assessed the frequency of colonic CD4+ T-cell subsets in both Il18−/− and Il18r1−/− mice. The frequency and total number of colonic CD4+ T cells was unaffected in Il18−/− or Il18r1−/− mice (see Supplementary Figure S2a). However, in the absence of IL-18R1 signaling the frequency of colonic effector/memory (CD62L−CD44+) CD4+ T cells was significantly increased and the frequency of naive (CD62L+CD44−) CD4+ T cells was significantly decreased (see Supplementary Figure S2b). Furthermore, absence of Il18 or Il18r1 resulted in significantly higher frequencies of colonic IL-17A and IFN-γ-producing CD4+ T cells compared with wild-type (WT) mice (Figure 2a,b). Expression of transcription factors Rorγt by IL-17A+ and T-bet by IFN-γ+ cells confirmed these populations as Th17 and Th1 cells, respectively (see Supplementary Figure S2c). By contrast, frequencies of Th17 and Th1 cells in the spleen and MLN were unaffected by Il18/Il18r1 deficiency (unpublished data). Elevated frequencies of colonic effector CD4+ T cells were not the result of a diminished Foxp3+ Treg cell population, as Foxp3+ Treg cell frequencies were equivalent between Il18−/−, Il18r1−/−, and WT mice (Figure 2c). Although IL-18-independent IL-18R1 signaling has been reported,21 the comparable increases of colonic Th17 and Th1 cell frequencies in Il18−/− and Il18r1−/− mice indicated that colonic effector CD4+ T cells are regulated by canonical IL-18/IL-18R1 signaling. In accordance with elevated frequencies of colonic Th17 cells, colonic lamina propria leukocytes isolated from Il18r1−/− mice and stimulated with α-CD3 produced significantly more IL-17A, but not granulocyte macrophage colony-stimulating factor or IL-22, when compared with WT controls (Figure 2d). By contrast, colonic lamina propria leukocytes isolated from Il18r1−/− mice did not produce significantly higher levels of IFN-γ ex vivo (Figure 2d), likely reflecting additional sources of IFN-γ besides Th1 cells. In addition, expression of genes encoding the antimicrobial peptides S100A8 and S100A9 induced by IL-17A, but not IL-22-induced Reg3γ, were significantly elevated in colonic tissue of Il18r1−/− mice when compared with WT controls (Figure 2e). The increased frequency of colonic Th17 cells observed in Il18−/− and Il18r1−/− mice was not due to elevated colonization by segmented filamentous bacteria as Il18−/−, Il18r1−/−, and WT mice had comparable segmented filamentous bacteria colonization (see Supplementary Figure S2d). Moreover, all three genotypes were co-housed for at least 3 weeks prior to analysis, in order to circumvent any potential effects of the commensal microbiota. Overall, these data show that canonical IL-18/IL-18R1 signaling limits colonic Th17 cell differentiation under homeostatic conditions.
Canonical interleukin (IL)-18R1 signaling limits colonic T helper type 17 (Th17) cell differentiation. Colonic lamina propria leukocytes from steady state, co-housed wild-type (WT), Il18−/−, and Il18r1−/− mice were analyzed for CD4+ T-cell subset frequencies. (a) Representative fluorescence-activated cell-sorted plots of IL-17A and interferon (IFN)-γ production by CD4+ T cells. Frequencies of (b) colonic Th1 and Th17 cells and (c) colonic Foxp3+ Treg cells from the indicated mice. (d) Cytokine production from α-CD3-stimulated colonic lamina propria leukocytes from WT and Il18r1−/− mice. (e) Antimicrobial peptide gene expression from colonic tissue of WT and Il18r1−/− mice. Data are shown as mean±s.e.m. and represents results from two to three independent experiments with consistent results. Each dot represents an individual mouse with n=4–5 mice/group. GM-CSF, granulocyte macrophage colony-stimulating factor.
IEC production of IL-18 limits colonic Th17 cell differentiation
We next sought to determine the cellular source of IL-18 within the steady-state intestine. Among the heterogeneous populations of intestinal myeloid cells, tissue-resident macrophages produce IL-1β upon microbial colonization to drive intestinal Th17 cell differentiation,7 and both IL-1β and IL-18 are dependent upon caspase-1 processing for their maturation.22 However, during dextran sulfate sodium–induced acute colitis, non-hematopoetic cell expression of caspase-1 appeared to mediate tissue protective responses that were associated with IL-18.18, 19 Furthermore, transcriptional analyses demonstrated that Il18 gene expression by IECs was elevated in the presence of commensal microbes.23 Thus we hypothesized that IECs might represent the major source of IL-18 within the homeostatic intestine. Accordingly, immunofluorescence analysis of colonic tissue identified extensive cytoplasmic IL-18 staining within E-cadherin+ IECs, whereas there were extremely few IL-18+ cells within the underlying lamina propria (Figure 3a). Complementary studies utilizing western blotting analysis of enriched colonic IECs and lamina propria leukocytes cell fractions confirmed this observation, with expression of mature IL-18 almost exclusively restricted to the IEC fraction (Figure 3b). Thus IECs represent the major source of IL-18 within the intestine under homeostatic conditions.
Intestinal epithelial cell (IEC)-derived interleukin (IL)-18 regulates colonic T helper type 17 (Th17) cell differentiation. (a) Representative confocal microscopic images of colonic tissue from wild-type (WT) and Il18−/− mice; IL-18 (red), E-cadherin (green), and 4,6-diamidino-2-phenylindole (DAPI; blue). Bar=50 μm. (b) Protein extracts from enriched IECs or colonic lamina propria leukocytes (cLPL) analyzed by western blotting with α-IL-18 and α-tubulin (loading control). Lethally irradiated WT and Il18−/− mice received WT or Il18−/− bone marrow cells to generate chimeric mice. (c) Representative fluorescence-activated cell-sorted plots of IL-17A and interferon (IFN)-γ production by colonic CD4+ T cells from chimeric mice. (d) Frequencies of colonic Th1, Th17, and Foxp3+ regulatory T cells from chimeric mice. Data represents results from two or three independent experiments. Each dot represents an individual mouse with n=4–5 mice/group.
To examine the role of IEC-derived IL-18 on colonic CD4+ T cells, we generated bone marrow (BM) chimeric mice by reconstituting lethally irradiated WT or Il18−/− mice with either WT or Il18−/− BM cells. Consistent with our findings in Il18−/− mice, we observed that Il18−/−→Il18−/− control chimeras had significantly increased frequencies of colonic Th17 cells compared with WT→WT controls (Figure 3c,d). Furthermore, selective ablation of Il18 in hematopoietic cells (Il18−/−→WT chimeras) did not alter colonic Th17 cell frequency compared with WT→WT controls, whereas selective ablation of Il18 in non-hematopoietic cells (WT→Il18−/− chimeras) resulted in significantly increased frequencies of colonic Th17 cells (Figure 3c,d). Conversely, in this chimeric setting, IEC-derived IL-18 slightly enhanced colonic Th1 cell differentiation, as WT→Il18−/− and Il18−/− →Il18−/− mice had slight reductions in colonic Th1 cell frequencies when compared with WT→WT control mice (Figure 3c,d). Expression of Rorγt by IL-17A+ and T-bet by IFN-γ+ cells confirmed these populations as Th17 and Th1 cells, respectively (see Supplementary Figure S3). In contrast to effector CD4+ T subsets, Foxp3+ Treg cell frequencies were unaffected by the absence of IEC-derived IL-18 (Figure 3d). Thus, under steady-state conditions, IECs are the primary source of constitutive IL-18 production in the gut, which acts to limit colonic Th17 cell differentiation.
Cell-intrinsic IL-18R1 signaling regulates colonic Th17 cell differentiation
To establish whether cell-intrinsic IL-18R1 signaling limited colonic Th17 cell differentiation, lethally irradiated Rag1−/− mice were adoptively transferred with a 1:1 mixture of BM cells from WT (CD45.1) and Il18r1−/− (CD45.2) donors. Equivalent reconstitution of WT and Il18r1−/− B220+ B cells, CD8+, and CD4+ T cells within the spleen, MLN, and colonic lamina propria indicated that Il18r1 deficiency did not generally affect lymphocyte reconstitution (see Supplementary Figure S4a). However, further analyses revealed that significantly higher frequencies of colonic Th17 cells were derived from Il18r1−/− CD4+ T cells when compared with WT CD4+ T cells (Figure 4a–c). Furthermore, IL-18R1 signaling limited Th17 cell differentiation solely within the colonic lamina propria, as Il18r1−/− CD4+ T cells and WT CD4+ T cells gave rise to comparable Th17 cell frequencies in the MLN and spleen (Figure 4c). In contrast, IL-18R1 signaling had a global role in promoting Th1 cell differentiation following lymphopenic expansion in this competitive chimeric environment, as significantly lower frequencies of Th1 cells were derived from Il18r1−/− CD4+ T cells in colonic lamina propria, MLN, and spleen (Figure 4c). However, frequencies of Foxp3+ Treg cells were again unaffected by Il18r1 deficiency throughout these chimeric mice (Figure 4c).
Cell-intrinsic interleukin (IL)-18R1 signaling inhibits colonic T helper type 17 (Th17) cell differentiation. Lethally irradiated Rag1−/− mice received a 1:1 mixture of CD45.1 wild-type (WT) and CD45.2 Il18r1−/− bone marrow cells. (a) Representative fluorescence-activated cell-sorted (FACS) plots of IL-17A and interferon (IFN)-γ production by colonic CD4+ T cells from chimeric mice. (b) Frequencies of colonic Th1, Th17, and Foxp3+ regulatory T (Treg) cells from chimeric mice. (c) Relative contribution of WT and Il18r1−/− CD4+ T cells to Th1, Th17, and Foxp3+ Treg cell pools in chimeric mice. Equal contribution=1, against which statistical significance was calculated using a one-column T-test. Ratio >1 indicates elevated contribution from Il18r1−/− progeny; ratio <1 indicates diminished contribution from Il18r1−/− progeny. (d) Representative FACS plots of IL-17A and IFN-γ production and (e) frequencies of Th17 cell differentiation by polarized WT or Il18r1−/− naive CD4+ T cells in the presence or absence of IL-18. (f) Representative FACS plots of IL-17A and IFN-γ production and (g) frequencies of Th17 cell differentiation by polarized WT, Il1r1−/− and Myd88−/− naive CD4+ T cells in the presence or absence of IL-18. Data are shown as mean±s.e.m. and represents results pooled from two to three independent experiments. Each dot represents an individual chimeric mouse with n=5–6 mice/group. MLN, mesenteric lymph node.
To investigate the mechanism by which IL-18 limits Th17 cell differentiation, we performed parallel in vitro studies. We utilized a Th17 cell differentiation system whereby co-culture of naive (CD62L+ CD44− CD25−) CD4+ T cells with BM-derived dendritic cells (BMDCs) in the presence of α-CD3, lipopolysaccharide, and transforming growth factor-β1 (TGFβ1) results in efficient Th17 cell polarization.24 Under these polarizing conditions, addition of IL-18 significantly inhibited Th17 cell differentiation from WT naive CD4+ T cells, whereas Il18r1−/− naive CD4+ T cells differentiated more efficiently into Th17 cells than WT naive CD4+ T cells, and this was not inhibited by addition of IL-18, confirming that IL-18 acts directly on CD4+ T cells to limit Th17 cell differentiation (Figure 4d,e). Expression of Rorγt by IL-17A-producing cells confirmed these as Th17 cells (see Supplementary Figure S4b), whereas T-bet and Foxp3 expression remained undetectable (unpublished data). Inhibition of Th17 cell differentiation by IL-18 appeared to occur independently of effects on cellular proliferation or apoptosis (see Supplementary Figure S4c,d). Together, these data confirm that IL-18 acts directly on CD4+ T cells to limit Th17 cell differentiation.
Both IL-1β and IL-18 signal through TIR-domain containing receptors that are dependent upon the myeloid differentiation primary response gene 88 (MyD88) signaling adaptor, in a manner akin to that of Toll-like receptors.25, 26 We therefore sought to address the seemingly opposing roles of IL-1β and IL-18 on Th17 cell differentiation. In line with previous reports,27 Il1r1−/− or Myd88−/− naive CD4+ T cells co-cultured with BMDCs under Th17 cell–polarizing conditions yielded significantly decreased frequencies of Th17 cells when compared with WT naive CD4+ T cells (Figure 4f,g). Strikingly, both IL-1R1 and MyD88 expression were required for IL-18-mediated inhibition of Th17 cell differentiation, as IL-18 significantly inhibited Th17 cell differentiation from WT CD4+ T cells but not from Il1r1−/− or Myd88−/− CD4+ T cells (Figure 4f,g). Thus IL-18 may limit Th17 cell differentiation by antagonizing MyD88-dependent signaling effectors downstream of IL-1R1. Together, these data demonstrate that IEC production of IL-18 acts via IL-18R1 directly on CD4+ T cells to regulate colonic Th17 cell differentiation.
CD4+ T-cell intrinsic IL-18R1 signaling is dispensable for induction of colitis
To assess the role of IL-18R1 signaling in induction of CD4+ T-cell-mediated intestinal inflammation, we utilized the T-cell transfer colitis model, whereby severe colitis and wasting disease are induced by reconstitution of lymphopenic hosts with naive CD4+ T cells.28 Initially, we sought to determine the cellular source of IL-18 during intestinal inflammation, as while IECs are a major source of IL-18 during homeostasis (Figure 3a), the key cellular source of IL-18 during chronic colitis remains unclear.20 Immunofluorescence analysis of colonic tissue from Rag1−/− mice adoptively transferred with WT naive CD4+ T cells demonstrated that IECs remain the major source of IL-18 within the inflamed colon, with IL-18 staining largely restricted to E-cadherin+ cells of the intestinal epithelium (Figure 5a). However, despite continued production of IL-18 by IECs during chronic colitis, we found equivalent levels of wasting disease and severe colitis in Rag1−/− recipients of WT or Il18r1−/− naive CD4+ T cells (Figure 5b,c). To assess the role of IL-18R1 signaling on colonic CD4+ T-cell differentiation in vivo under inflammatory conditions, we isolated colonic lamina propria leukocytes from Rag1−/− recipients that received WT or Il18r1−/− naive CD4+ T cells and identified Th1, Th17, and Foxp3+ T-cell populations by flow cytometry. We observed similar frequencies of IL-17A+ Th17 and IL-17A+ IFN-γ+ double-producing CD4+ T cells in the inflamed colons of Rag1−/− mice that received WT or Il18r1−/− naive CD4+ T cells (see Supplementary Figure S5a,b). Furthermore, although the frequencies of IFN-γ+ Th1 cells were slightly decreased in the colons of Rag1−/− mice receiving Il18r1−/− naive CD4+ T cells when compared with those receiving WT naive CD4+ T cells, this was not statistically significant (see Supplementary Figure S5a,b). Finally, the differentiation of small numbers of Foxp3+-induced Treg cells that occurs in this model29 was not dependent upon IL-18R1 signaling, as Rag1−/− recipient mice receiving WT or Il18r1−/− naive CD4+ T cells had equivalent frequencies of colonic Foxp3+-induced Treg cells (see Supplementary Figure S5c). Together, these data indicate that CD4+ T-cell intrinsic IL-18R1 signaling is dispensable for induction of intestinal inflammation upon adoptive transfer of naive CD4+ T cells into a lymphopenic environment.
Interleukin (IL)-18R1 signaling is dispensable for induction of CD4+ T-cell-mediated colitis. Rag1−/− mice received wild-type (WT) or Il18r1−/− naive CD4+ T cells. (a) Representative confocal microscopic images of colonic tissue from Rag1−/− mice receiving WT naive CD4+ T cells. IL-18 (red), E-cadherin (green) and 4,6-diamidino-2-phenylindole (DAPI; blue). Bar=50 μm. (b) Representative weight loss curves and (c) colonic inflammation scores of Rag1−/− mice alone or receiving WT or Il18r1−/− naive CD4+ T cells. Data shown as mean are representative of two independent experiments. Each dot represents an individual chimeric mouse with n=4–5 mice/group.
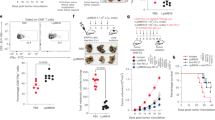
Canonical IL-18R1 signaling is critical for Foxp3+ Treg cell–mediated control of colitis
Although colonic Foxp3+ Treg cells expressed IL-18R1 during homeostasis (Figure 1d), IL-18R1 deficiency did not alter the frequency of this cellular population (Figure 2c). However, we hypothesized that IL-18R1 signaling on Foxp3+ Treg cells may influence their function within the colonic lamina propria. To address this question, we utilized a modification of the T-cell transfer colitis model, whereby co-transfer of Foxp3+ Treg cells prevents naive CD4+ T-cell-mediated wasting disease and intestinal inflammation.30 To ensure Foxp3+ Treg cell purity and facilitate detection of IL-10 production by Foxp3+ Treg cells, we crossed Il18r1−/− mice with Foxp3hCD2 mice31 and IL-10gfp mice32 to generate WT and Il18r1−/− Foxp3hCD2 IL-10gfp mice, from which Foxp3+ Treg cells were isolated based on hCD2 expression. First, we analyzed IL-18R1 expression by Foxp3+ Treg cells during control of colitis in Rag1−/− mice that received both WT naive CD4+ T cells and WT Foxp3+ CD4+ T cells. Four weeks post-transfer, 86.0±8.1% of colonic Foxp3+ Treg cells were IL-18R1+ (Figure 6a,b), suggesting a functional role for IL-18R1 signaling in control of colitis. To directly address this, Rag1−/− mice received WT naive CD4+ T cells alone or in combination with WT or Il18r1−/− Foxp3+ Treg cells (CD45.2). As expected, co-transfer of WT Foxp3+ Treg cells completely prevented wasting disease and colitis induced by naive CD4+ T cells (Figure 6c–e). By contrast, Rag1−/− mice receiving naive CD4+ T cells and Il18r1−/− Foxp3+ Treg cells developed wasting disease and severe intestinal inflammation (Figure 6c–e). Rag1−/− mice receiving Il18r1−/− Foxp3+ Treg cells alone did not develop colitis, indicating that Il18r1−/− Foxp3+ Treg cells do not acquire pathogenic potential upon transfer to lymphopenic hosts (Figure 6c–e). Importantly, we observed equivalent levels of reconstitution and maintenance of Foxp3 expression by both WT and Il18r1−/− Foxp3+ Treg cells (Figure 6f). Furthermore, Foxp3+ IL-10+ Treg cell frequencies were elevated in the absence of IL-18R1 signaling (Figure 6f), likely in response to the on-going intestinal inflammation (Figure 6d,e). Thus the inability of Il18r1−/− Foxp3+ Treg cells to suppress colitis was not due to inefficient reconstitution, loss of Foxp3 expression, or failure to produce IL-10.
Canonical interleukin (IL)-18R1 signaling is critical for Foxp3+ regulatory T (Treg) cell–mediated control of colitis. Rag1−/− mice received CD45.1+ naive CD4+ T cells alone or in combination with CD45.2+ wild-type (WT) or Il18r1−/− Foxp3+ Treg cells. (a) Representative fluorescence-activated cell-sorted analysis and (b) percentages of colonic Foxp3+ Treg cell expression of IL-18R1 from Rag1−/− mice receiving WT naive CD4+ T cells and Foxp3+ Treg cells. (c) Representative weight loss curves, (d) histological sections, and (e) colonic inflammation scores of Rag1−/− mice receiving CD45.1+ naive CD4+ T cells alone, in combination with WT or Il18r1−/− Foxp3+ Treg cells, or Il18r1−/− Foxp3+ Treg cells alone. (f) Frequencies of colonic CD45.2+ T cells, expressing Foxp3, and producing IL-10, from Rag1−/− mice receiving CD45.1+ naive CD4+ T cells in combination with WT or Il18r1−/− Foxp3+ Treg cells. (g) Quantitative reverse transcriptase–PCR validation of differential gene expression by WT and Il18r1−/− Foxp3+ Treg cells from Rag1−/− mice receiving CD45.1+ naive CD4+ and CD25+ Treg cells in combination with CD45.2+ WT or Il18r1−/− Foxp3+ Treg cells. Data are shown as mean±s.e.m. and represents results from two to three independent experiments. Each dot represents an individual mouse with n=2–6 mice/group.
Canonical IL-18R1 signaling promotes Foxp3+ Treg cell effector molecule expression
We next characterized the effects of IL-18R1 signaling on colonic Foxp3+ Treg cells by comparing the gene expression profiles of WT and Il18r1−/− Foxp3+ Treg cells during prevention of colitis. Thus Rag1−/− mice received WT naive CD4+ T cells and WT CD4+ CD25+ Treg cells (both CD45.1) together with either WT or Il18r1−/− Foxp3+ Treg cells (CD45.2). Four weeks posttransfer, colonic CD45.2+ WT or Il18r1−/− Foxp3+ Treg cells were isolated, and comparative whole transcriptome analysis was performed. Among transcripts differentially expressed by Il18r1−/− Foxp3+ Treg cells, we identified 1606 and 2153 probes to be significantly elevated or decreased, respectively, compared with WT controls (see Supplementary Figure S6a). Although differentially expressed transcripts showed consistent clustering across the Il18r1−/− Foxp3+ Treg cell samples, we did not identify any significantly enriched pathways using Gene Ontology (GO) or KEGG analysis. Nonetheless, among transcripts positively regulated by IL-18R1 signaling, we identified numerous genes associated with the Foxp3+ Treg cell signature and effector functions (see Supplementary Figure S6a). Subsequent quantitative reverse transcriptase–PCR analysis verified significantly lower expression of the majority of these genes by Il18r1−/− Foxp3+ Treg cells (Figure 6g). Furthermore, these validated transcripts included multiple protein-coding genes previously demonstrated as being critical for suppression of T-cell-mediated colitis by Treg cells, including Furin, Bcl11b, Tnfrsf4, and Stat3.33, 34, 35, 36 Finally, canonical IL-18-dependent IL-18R1 signaling promoted gene expression changes in Foxp3+ Treg cells, as acute stimulation of Foxp3+ Treg cells with IL-18 resulted in elevated expression of a number of these transcripts (see Supplementary Figure S6b). Taken together, these results indicate that, although IL-18R1 signals are not required for Foxp3+ Treg cell differentiation, they promote optimal Foxp3+ Treg cell function within the colonic lamina propria by enhancing the expression of key Foxp3+ Treg cell effectors.
DISCUSSION
Accumulating evidence demonstrates key roles for IL-1 family cytokines in promoting effector and regulatory CD4+ T-cell responses at mucosal barrier surfaces. IL-1β promotes Th17 cell differentiation under homeostatic and inflammatory conditions, while IL-33 enhances colonic Foxp3+ Treg cell function to limit intestinal inflammation.7, 37, 38 We now identify complementary roles for IL-18 in limitation of homeostatic Th17 cell differentiation and in promotion of Foxp3+ Treg cell function to limit tissue pathology during experimental colitis. In addition, our findings place canonical IL-18/IL-18R1 signaling at the center of a key epithelial/T-cell immune-regulatory axis in the intestine.
Basal activation of innate immune pathways at mucosal surfaces, particularly within the gastrointestinal tract, is key for maintenance of tissue homeostasis. The commensal dysbiosis and elevated susceptibility to acute experimental dextran sulfate sodium colitis observed in mice lacking Nod-like receptor components demonstrates the crucial role of inflammasome activation in maintenance of intestinal homeostasis.18, 19, 39 The protective effects of inflammasome activation in the intestine have been partly attributed to the production of IL-18, which may be important in restoring and maintaining epithelial barrier integrity.18, 19 Conversely, evidence for a pathogenic role for IL-18 in experimental CD4+ T-cell-mediated colitis,17 together with elevated tissue levels of IL-18 reported in patients with IBD,15, 16 suggest that this cytokine may contribute to chronic intestinal inflammation. However, little is known of the direct cellular targets of IL-18 within the gastrointestinal tract, nor the downstream consequences of this interaction.20 We focused our investigation to the modulation of intestinal CD4+ T-cell populations by IL-18, and the functional activities of this pathway during intestinal homeostasis and inflammation. We observed high IL-18R1 expression by effector/memory CD4+ T cells, particularly within the colonic lamina propria, reflective of the elevated activation status of intestinal CD4+ T cells.40 However, we observed discordant levels of IL-18R1 expression by distinct effector and regulatory intestinal CD4+ T-cell subsets, with highest expression by colonic Th17 cells. Local exposure of colonic Th17 cells to IL-23 may bolster IL-18R1 expression, as Il18r1 expression is positively regulated by IL-23 signaling in developing Th17 cells.41
Although our results indicated that colonic CD4+ T cells were poised to respond to IL-18 under homeostatic conditions, the cellular source of this cytokine remained unclear. Using both immunofluorescence and western blotting, we observed production of IL-18 from E-cadherin+ IECs during homeostatic conditions, consistent with previous reports that a non-hematopoietic source of IL-18 promotes tissue-protective responses during acute dextran sulfate sodium colitis.18, 19 Interestingly, IL-18 production appeared restricted to IECs outside of the crypt base, suggesting that IL-18 production is limited to mature epithelial cells or that IL-18 production is triggered in response to microbial stimulation outside of the relatively sterile crypt base. The factors that regulate IL-18 production by IECs require additional investigation, but it has been reported that commensal microbes may augment Il18 gene expression by IECs.23 Homeostatic production of IL-18 by IECs and high IL-18R1 expression by colonic CD4+ T cells led us to investigate the role of this axis in immune regulation in the steady-state intestine. Notably, absence of canonical IL-18R1 signaling resulted in elevated frequencies of colonic Th17 cells under homeostatic conditions. Although expression of IL-18R1 by colonic Th17 cells suggested that IL-18 could act directly on this cell population to limit their differentiation, we also considered the possibility that the absence of functional IL-18R1 signaling in another leukocyte population (for example, in Foxp3+ Treg cells) may have indirectly led to the increased frequency of colonic Th17 cells. However, competitive BM chimera experiments demonstrated that cell-intrinsic IL-18R1 signaling acted to limit colonic Th17 cell differentiation. Thus, IEC-derived IL-18 acts directly on colonic CD4+ T cells to limit Th17 cell differentiation under homeostatic conditions. Like Toll-like receptors, the IL-1 family cytokines IL-1β and IL-18 signal through TIR-domain containing receptors, dependent upon the signaling adaptor, MyD88.25 In accordance with a cell-intrinsic role for canonical IL-18R1 signaling in limiting Th17 cell differentiation, addition of IL-18 decreased the efficiency of Th17 cell differentiation by acting directly on CD4+ T cells. However, CD4+ T-cell expression of IL-1R1 or MyD88 was required for IL-18 to limit Th17 cell differentiation, suggesting that IL-18 limits Th17 cell differentiation in part by antagonizing signaling downstream of IL-1R1. Future work is necessary to better understand how distinct MyD88-dependent signaling cascades can direct opposing transcriptional responses, an area that remains poorly understood despite extensive investigation in the context of Toll-like receptor signaling.
In contrast to the clear inhibitory effects of IL-18R1 signaling on intestinal Th17 differentiation, we observed opposing effects of IL-18R1 ablation on colonic Th1 cell development under homeostatic conditions and in the competitive BM chimeras. Under steady-state conditions, IL-18R1 signaling limited Th1 differentiation, evidenced by the increased frequencies of Th1 cells found in Il18−/− and Il18r1−/− mice compared with WT mice. However, in the BM chimeric mice IL-18 promoted Th1 cell differentiation, as Il18r1−/− CD4+ T cells had reduced frequencies of Th1 cells relative to WT CD4+ T cells. We hypothesize that intestinal epithelial barrier damage and lymphopenia-induced proliferation following irradiation42 creates an inflammatory environment in the BM chimeric mice, in which IL-18 has a more classical role in augmenting Th1 cell responses.8 Indeed, the overall frequencies of effector CD4+ T cells found in the intestines of the BM chimeras were markedly higher than those observed in steady-state mice. However, we cannot exclude the possibility of a cell-extrinsic role for IL-18R1 signaling, perhaps through Foxp3+ Treg cell function, in control of colonic Th1 cell differentiation under homeostatic conditions.
We also addressed the role of the IL-18/IL-18R1 signaling axis in the induction and regulation of CD4+ T-cell-mediated intestinal inflammation. Our experiments suggested that IECs remained a key source of IL-18 during experimental CD4+ T-cell-mediated intestinal inflammation, as we observed robust IL-18 staining in E-cadherin+ IECs and very few IL-18+ cells within the lamina propria compartment. This observation contrasts with findings from patients with Crohn’s disease, where the increased levels of IL-18 present in inflamed lesions coincided with increased accumulation of IL-18+ CD68+ macrophages in the lamina propria.16, 43 These observations may reflect some of the inherent differences between the inflammatory characteristics of experimental T-cell-transfer colitis in mice and Crohn’s disease. However, a comprehensive investigation into the cellular sources of bioactive IL-18 within the human intestine during health and disease could help explain its context-dependent protective or pathogenic activities. Although IECs remained a major source of IL-18 during colitis, IL-18R1 signaling on CD4+ T cells was dispensable for induction of intestinal inflammation, as Rag1−/− recipients of Il18r1−/− naive CD4+ T cells developed colitis of equivalent severity and with similar kinetics as those that received WT naive CD4+ T cells. A previous study provided evidence of a pathogenic role for IL-18 in the effector phase of T-cell-transfer colitis, as administration of a recombinant adenovirus expressing IL-18 antisense mRNA to mice with established colitis led to a reduction in intestinal pathology.17 Although this appears to conflict with our findings, a lack of requirement for T-cell intrinsic IL-18R1 signals for disease induction does not preclude a contribution of IL-18 to the perpetuation of established inflammation. Indeed, we found that, during severe inflammation in the colonic lamina propria, IL-18R1 signaling no longer limited Th17 cell differentiation and slightly, but not significantly, enhanced Th1 cell differentiation. This indicates that the effects of IL-18 on intestinal T-cell responses may vary depending on local conditions within the gut.
Finally, we found that, although IL-18R1 signals were dispensable for the differentiation of intestinal Foxp3+ Treg cells during steady state, expression of IL-18R1 by Foxp3+ Treg cells was essential for Treg cell suppression of intestinal inflammation in the T-cell-transfer colitis model. Mechanistic analyses demonstrated that although IL-18R1 signaling did not affect Treg cell reconstitution, maintenance of Foxp3 expression, or IL-10 production, canonical IL-18R1 signaling promoted the expression of a number of key Foxp3+ Treg cell effector molecules previously demonstrated to be critical for control of colitis by Treg cells, including Furin, Bcl11b, Tnfrsf4, and Stat3.33, 34, 35, 36 In the context of IBD, elevated levels of IL-18 in intestinal tissues have been proposed to contribute to immunopathology by promoting effector Th1 cell responses. However, our results suggest that, during intestinal inflammation, IEC-derived IL-18 contributes to a negative feedback loop to regulate intestinal inflammation by promoting optimal function of Foxp3+ Treg cells. Thus IL-18 acts as a tissue-specific modifying factor that boosts intestinal Foxp3+ Treg cell effector function. Of relevance to clinical settings, polymorphisms within the IL18R1–IL18RAP locus leading to decreased IL-18R component expression have been identified by genome-wide association studies to be associated with both adult and early-onset IBD.12, 13, 14 In light of our results, it is tempting to speculate that attenuated canonical IL-18R1 signaling in intestinal Treg cell may provide a potential mechanistic basis for the link between common variants in the IL18R1–IL18RAP locus and susceptibility to both adult and early-onset IBD. In summary, our results place IL-18 at the center of a regulatory axis through which IECs modulate local T-cell responses in the gut. They further suggest that strategies targeting IL-18 in the context of IBD should be employed with caution, as they may disrupt important regulatory circuits that act to limit intestinal inflammation.
METHODS
Mice. WT (C57BL/6), B6.SJL-CD45.1 (CD45.1), B6 Rag1−/−, B6 Il18−/−, B6 Il18r1−/−, B6 Foxp3hCD2, and B6 IL-10gfp mice were maintained under specified pathogen free conditions in accredited facilities at the University of Oxford, Oxford, UK. B6 Il18r1−/−, Foxp3hCD2 and IL-10gfp mice were crossed to generate B6 Il18r1−/− Foxp3hCD2 IL-10gfp mice. All procedures on mice were conducted in accordance with the UK Scientific Procedures Act (1986) under a project license (PPL) authorized by the UK Home Office Animal Procedures Committee and approved by the Sir William Dunn School of Pathology Local Ethical Review Committee. Mice were routinely screened for Helicobacter species and were 6 weeks of age when first used.
CD4+ T-cell purification. Bulk CD4+ T cells were purified from the spleen and peripheral lymph nodes by negative selection. Naive CD4+ T cells for in vivo use were sorted as CD4+ CD25− CD45RBHi, while regulatory T cells were sorted as CD4+ hCD2+ or CD4+ CD25+. Naive T cells for in vitro use were sorted as CD4+ CD25− CD44− CD62L+. Cells were sorted on a MoFlo (Dako, Glostrup, Denmark) or AriaIII (BD Bioscience, San Jose, CA), routinely to >99% purity.
T-cell transfer model of colitis. Naive CD4+ CD25− CD45RBHi T cells from CD45.1, WT, or Il18r1−/− mice were transferred via intraperitoneal injection to sex-matched B6 Rag1−/− recipient mice (4 × 105 cells per mouse). WT or Il18r1−/− CD4+ Foxp3+ Treg cells were co-transferred in the same intraperitoneal injection where indicated (2 × 105 cells per mouse). For transcriptome analysis, 2 × 105 CD45.1 CD4+ CD25+ cells were also transferred in order to prevent intestinal inflammation in hosts receiving Il18r1−/− Foxp3+ Treg cells. Mice were monitored weekly for wasting disease, and mice losing >20% initial weight or developing clinical signs of colitis were euthanized.
Histological assessment of intestinal inflammation. Histological analysis of colitis was performed as described.44 Briefly, mice were euthanized 4–7 weeks following T-cell transfer and samples of proximal, middle, and distal colon were fixed in 10% formalin. Paraffin-embedded samples were cut into 4-μm sections and hematoxylin and eosin stained, and inflammation was scored in a blinded manner. Inflammation was graded semi-quantitatively on a scale from 0 to 3, for four criteria; (a) epithelial hyperplasia and goblet cell depletion, (b) lamina propria leukocyte infiltration, (c) area of tissue affected, and (d) markers of severe inflammation, including crypt abscesses, sub-mucosal inflammation, and ulceration. Scores for individual criteria were totaled for an overall inflammation score between 0 and 12.
Generation of BM chimeras. BM cells were isolated from CD45.1, WT, Il18−/−, or Il18r1−/− mice and transferred singularly or mixed at 1:1 ratio (as indicated) into lethally irradiated (1100 Rad, split dose) recipients via intravenous injection (a total of 1 × 107 cells per mouse). Mice were allowed to reconstitute for >6 weeks before analysis.
Isolation of cells and fluorescence-activated cell sorting analysis. Cell suspensions were prepared from spleen, MLN, colonic lamina propria, and BM as previously described.34 Enriched IEC fractions were isolated by incubation of total colonic tissue in RPMI 1640, 5% fetal calf serum, and 25 mM EDTA for 15 min before collection of liberated IECs. All antibodies for flow cytometry were from eBioscience (Hatfield, UK), apart from α-IL-18R1 (R&D Systems, Minneapolis, MN). Data were acquired using a Cyan ADP (Beckman Coulter, High Wycombe, UK) or BD Fortessa (BD Bioscience) and analyzed using the FlowJo software (Tree Star, Ashland, OR).
Th17 cell polarization with BMDCs. Naive CD4+ T-cell and dendritic cell co-culture was adopted from studies reported previously.24 Briefly, fluorescence-activated cell-sorted naive CD4+ CD25− CD44− CD62L+ T cells (2.5 × 105) from WT, Il18r1−/−, Il1r1−/−, or Myd88−/− mice were activated with 1 μg ml−1 soluble α-CD3 (clone 145-2C11) and BMDCs from WT mice (1 × 105) in the presence of 1 ng ml−1 TGFβ1, 100 ng ml−1 lipopolysaccharide, α-IFN-γ, α-IL-4 (both 10 μg ml−1) ±IL-18 (both 10 ng ml−1).
Polarization and stimulation of CD4+ T-cell subsets. Naive CD4+ T cells cultured on α-CD3/CD28 were activated with 5 μg ml−1 α-CD3 and 2 μg ml−1 α-CD28 for Th0 or in the presence of IL-12 (10 ng ml−1), α-IL-4 (10 μg ml−1) for Th1, IL-4 (20 ng ml−1), α-IFN-γ (10 μg ml−1) for Th2, TGF-β1 (250 pg ml−1), IL-1β (10 ng ml−1), IL-6 (20 ng ml−1), IL-23 (10 ng ml−1), α-IFN-γ, α-IL-4 (both 10 μg ml−1) for Th17, and TGF-β1 (5 ng ml−1), IL-2 (100 U ml−1) α-IFN-γ, α-IL-4 (both 10 μg ml−1) for induced Treg polarization. Cells were cultured in Iscove’s Modified Dulbecco’s Medium, 10% fetal calf serum, 2 mM L-glutamine, 100 U ml−1 of Penicillin/Streptomycin, and 0.05 mM 2-mercaptoethanol in 1 ml media in a 48-well plate. Sorted Foxp3+ Treg cells were activated for 24 h with 5 μg ml−1 α-CD3 and 2 μg ml−1 α-CD28 and then stimulated for 45 min with media or IL-18 (10 ng ml−1).
Immunofluorescence. Colonic tissue samples were formalin-fixed, paraffin-embedded and sectioned as per histological analysis. Sections were deparaffinized, rehydrated, and subjected to sodium citrate–based antigen retrieval, then stained with rabbit α-IL-18 (Abcam, Milton, UK), mouse α-E-cadherin (BD Bioscience), goat α-rabbit Alexa-555 and goat α-mouse Alexa488 (Life Technologies, Paisley, UK), and counterstained with DAPI (4,6-diamidino-2-phenylindole). Images were acquired with an Olympus Fluoview FV1000 confocal microscope and Olympus Fluoview Software (Olympus, Tokyo, Japan).
Quantitation of gene expression. Total RNA from tissue samples or frozen cells was purified using the RNAeasy Kits (Qiagen, Hilden, Germany). Tissue homogenization was performed using a FastPrep 24 Homogenizer (MP Biomedicals, Burlingame, CA). cDNA was synthesized using the Superscript III reverse transcriptase and Oligo d(T) primers (Invitrogen, Carlsbad, CA). Reactions were assayed in triplicate on a Chromo4 detection system (MJ Research, Waltham, MA), with expression levels for individual samples normalized relative to Hprt1 (Qiagen).
Transcriptome analysis. Whole transcriptome analysis was performed with 2–3 biological replicates per experimental group, with each sample pooled from 4 mice. Total RNA was purified using the RiboPure Kit (Ambion, Austin, TX) and amplified using the TargetAmp 2-Round Biotin-aRNA Amplification Kit (Cambio, Cambridge, UK), and whole genome expression profiled using the Single-Color MouseWG-6_V2BeadChip with direct hybridization assay (Illumina, San Diego, CA). Cy3 fluorscence emissions were imaged using iScan system (Illumina). Signal intensities generated using the GenomeStudio 2011 software (Illumina) were background corrected and subjected to variance stabilizing transformation implemented in the Lumi package from R/Bioconductor.45 Quantile-normalized signal intensities were used for differential expression analysis implemented in Limma.46 Probes below a Benjamini–Hochberg-derived adjusted P-value of 0.1 were deemed significant. Microarray data are deposited with ArrayExpress and available under accession code E-MTAB-3067.
Western blotting analysis. Total protein extracts were generated by homogenizing snap-frozen colonic tissue in RIPA buffer containing protease inhibitor cocktail (Roche, Basel, Switzerland). Protein levels were normalized by Lowry assay (Bio-Rad Laboratories, Hercules, CA), resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis nd analyzed with α-IL-18 (Abcam, ab71495) and α-tubulin (Santa Cruz Biotechnology, Dallas, TX; sc5286).
Statistical analysis. Statistical analysis was determined by two-way analysis of variance with Bonferroni post-test for weight curves. The Mann–Whitney test was utilized for all in vivo experiments, except where a one-column T-test with a hypothetical value of 1 was employed. In vitro experiments were analyzed by Student’s T-test (P<0.05; *P<0.05, **P<0.01, ***P<0.001).
References
Hooper, L.V., Littman, D.R. & Macpherson, A.J. Interactions between the microbiota and the immune system. Science 336, 1268–1273 (2012).
Maloy, K.J. & Powrie, F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 474, 298–306 (2011).
Korn, T., Bettelli, E., Oukka, M. & Kuchroo, V.K. IL-17 and Th17 cells. Annu. Rev. Immunol. 27, 485–517 (2009).
Harrison, O.J. & Powrie, F.M. Regulatory T cells and immune tolerance in the intestine. Cold Spring Harb. Perspect. Biol. 5 pii: a018341 (2013).
Atarashi, K. et al. ATP drives lamina propria T(H)17 cell differentiation. Nature 455, 808–812 (2008).
Ivanov, I.I. et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 4, 337–349 (2008).
Shaw, M.H., Kamada, N., Kim, Y.G. & Nunez, G. Microbiota-induced IL-1beta, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine. J. Exp. Med. 209, 251–258 (2012).
Sims, J.E. & Smith, D.E. The IL-1 family: regulators of immunity. Nat. Rev. Immunol. 10, 89–102 (2010).
Okamura, H. et al. A novel costimulatory factor for gamma interferon induction found in the livers of mice causes endotoxic shock. Infect. Immun. 63, 3966–3972 (1995).
Robinson, D. et al. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-gamma production and activates IRAK and NFkappaB. Immunity 7, 571–581 (1997).
Smeltz, R.B., Chen, J., Hu-Li, J. & Shevach, E.M. Regulation of interleukin (IL)-18 receptor alpha chain expression on CD4(+) T cells during T helper (Th)1/Th2 differentiation. Critical downregulatory role of IL-4. J. Exp. Med. 194, 143–153 (2001).
Barrett, J.C. et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nat. Genet. 40, 955–962 (2008).
Hedl, M., Zheng, S. & Abraham, C. The IL18RAP region disease polymorphism decreases IL-18RAP/IL-18R1/IL-1R1 expression and signaling through innate receptor-initiated pathways. J. Immunol. 192, 5924–5932 (2014).
Imielinski, M. et al. Common variants at five new loci associated with early-onset inflammatory bowel disease. Nat. Genet. 41, 1335–1340 (2009).
Monteleone, G. et al. Bioactive IL-18 expression is up-regulated in Crohn's disease. J. Immunol. 163, 143–147 (1999).
Pizarro, T.T. et al. IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn's disease: expression and localization in intestinal mucosal cells. J. Immunol. 162, 6829–6835 (1999).
Wirtz, S., Becker, C., Blumberg, R., Galle, P.R. & Neurath, M.F. Treatment of T cell-dependent experimental colitis in SCID mice by local administration of an adenovirus expressing IL-18 antisense mRNA. J. Immunol. 168, 411–420 (2002).
Dupaul-Chicoine, J. et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity 32, 367–378 (2010).
Zaki, M.H. et al. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 32, 379–391 (2010).
Siegmund, B. Interleukin-18 in intestinal inflammation: friend and foe? Immunity 32, 300–302 (2010).
Gutcher, I., Urich, E., Wolter, K., Prinz, M. & Becher, B. Interleukin 18-independent engagement of interleukin 18 receptor-alpha is required for autoimmune inflammation. Nat. Immunol. 7, 946–953 (2006).
Schroder, K. & Tschopp, J. The inflammasomes. Cell 140, 821–832 (2010).
Pott, J. et al. Age-dependent TLR3 expression of the intestinal epithelium contributes to rotavirus susceptibility. PLoS Pathogens 8, e1002670 (2012).
Veldhoen, M., Hocking, R.J., Atkins, C.J., Locksley, R.M. & Stockinger, B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006).
Adachi, O. et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9, 143–150 (1998).
O'Neill, L.A. & Bowie, A.G. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 7, 353–364 (2007).
Chung, Y. et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity 30, 576–587 (2009).
Powrie, F., Leach, M.W., Mauze, S., Caddle, L.B. & Coffman, R.L. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int. Immunol. 5, 1461–1471 (1993).
Izcue, A. et al. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity 28, 559–570 (2008).
Hori, S., Nomura, T. & Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 (2003).
Komatsu, N. et al. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc. Natl. Acad. Sci. USA 106, 1903–1908 (2009).
Kamanaka, M. et al. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity 25, 941–952 (2006).
Chaudhry, A. et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science 326, 986–991 (2009).
Griseri, T., Asquith, M., Thompson, C. & Powrie, F. OX40 is required for regulatory T cell-mediated control of colitis. J. Exp. Med. 207, 699–709 (2010).
Pesu, M. et al. T-cell-expressed proprotein convertase furin is essential for maintenance of peripheral immune tolerance. Nature 455, 246–250 (2008).
Vanvalkenburgh, J. et al. Critical role of Bcl11b in suppressor function of T regulatory cells and prevention of inflammatory bowel disease. J. Exp. Med. 208, 2069–2081 (2011).
Coccia, M. et al. IL-1beta mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. J. Exp. Med. 209, 1595–1609 (2012).
Schiering, C. et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 513, 564–568 (2014).
Elinav, E. et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145, 745–757 (2011).
Carpenter, A.C. et al. The transcription factors Thpok and LRF are necessary and partly redundant for T helper cell differentiation. Immunity 37, 622–633 (2012).
Ghoreschi, K. et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature 467, 967–971 (2010).
Paulos, C.M. et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J. Clin. Invest. 117, 2197–2204 (2007).
Kanai, T. et al. Macrophage-derived IL-18-mediated intestinal inflammation in the murine model of Crohn's disease. Gastroenterology 121, 875–888 (2001).
Song-Zhao, G.X. & Maloy, K.J. Experimental mouse models of T cell-dependent inflammatory bowel disease. Methods Mol. Biol. 1193, 199–211 (2014).
Du, P., Kibbe, W.A. & Lin, S.M. lumi: a pipeline for processing Illumina microarray. Bioinformatics 24, 1547–1548 (2008).
Smyth, G.K. In Bioinformatics and Computational Biology Solutions Using R and Bioconductor Statistics for Biology and Health Gentleman R., et al., eds 397–420 Springer: New York, NY, (2005).
Acknowledgements
We thank Helen Ferry, Kate Alford, and Nigel Rust for cell sorting; Richard Stillion for histology; staff at the University of Oxford for animal care; High Throughput Genomics; The Wellcome Trust Centre for Human Genetics for microarray services; and Professor Herman Waldmann and Professor Richard Flavell for Foxp3hCD2 and IL-10gfp mice, respectively. We are grateful for support from the Wellcome Trust (New Investigator Award to K.J.M.; Doctoral studentship to O.J.H).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Rights and permissions
About this article
Cite this article
Harrison, O., Srinivasan, N., Pott, J. et al. Epithelial-derived IL-18 regulates Th17 cell differentiation and Foxp3+ Treg cell function in the intestine. Mucosal Immunol 8, 1226–1236 (2015). https://doi.org/10.1038/mi.2015.13
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2015.13
This article is cited by
-
Regulatory T cells in the face of the intestinal microbiota
Nature Reviews Immunology (2023)
-
Enterocyte–innate lymphoid cell crosstalk drives early IFN-γ-mediated control of Cryptosporidium
Mucosal Immunology (2022)
-
The deubiquitinase USP25 supports colonic inflammation and bacterial infection and promotes colorectal cancer
Nature Cancer (2020)
-
Telomere dysfunction activates YAP1 to drive tissue inflammation
Nature Communications (2020)
-
Inflammasome inhibitors: promising therapeutic approaches against cancer
Journal of Hematology & Oncology (2019)