Abstract
We examined the function of the oxysterol receptors (LXRs) in inflammatory bowel disease (IBD) through studying dextran sodium sulfate (DSS)- and 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis in mice and by elucidating molecular mechanisms underlying their anti-inflammatory action. We observed that Lxr-deficient mice are more susceptible to colitis. Clinical indicators of colitis including weight loss, diarrhea and blood in feces appeared earlier and were more severe in Lxr-deficient mice and particularly LXRβ protected against symptoms of colitis. Addition of an LXR agonist led to faster recovery and increased survival. In contrast, Lxr-deficient mice showed slower recovery and decreased survival. In Lxr-deficient mice, inflammatory cytokines and chemokines were increased together with increased infiltration of immune cells in the colon epithelium. Activation of LXRs strongly suppressed expression of inflammatory mediators including TNFα. While LXRα had anti-inflammatory effects in CD11b+ immune cell populations, LXRβ in addition had anti-inflammatory effects in colon epithelial cells. Lack of LXRβ also induced CD4+/CD3+ immune cell recruitment to the inflamed colon. Expression of both LXRA and LXRB was significantly suppressed in inflamed colon from subjects with IBD compared with non-inflamed colon. Taken together, our observations suggest that the LXRs could provide interesting targets to reduce the inflammatory responses in IBD.
Similar content being viewed by others
Introduction
The oxysterol receptors (Liver X receptors; LXRs) encoded by the genes Nhr1h3 (LXRα) and Nhr1h2 (LXRβ) are members of the nuclear receptor family and established ligand-regulated transcription factors involved in many metabolic processes including lipid, cholesterol, and carbohydrate metabolism.1, 2 LXRs were also reported to inhibit the expression of several inflammatory mediators in macrophages,3 a mechanism later shown to involve post-translational modification of LXRs via SUMOylation.4 Considerable evidence has since emerged identifying LXRs as both anti-inflammatory transcription factors and physiological regulators of innate and adaptive immune responses, apoptosis, and phagocytosis.1, 2
Several studies have proposed a protective role of LXRs in various models of diseases with an inflammatory component. Activation of LXRs decreased the severity and development of experimental autoimmune encephalomyelitis,5, 6, 7 a rodent experimental model of multiple sclerosis. Apparently, the protective effect was due to the suppression of differentiation of TH-17 cells, often associated with MS, via induced transcription of the lipogenic sterol regulatory element-binding protein (Srebp1c) LXR target gene.7 Srebp1c bound to the promoter of the interleukin 17 (Il17) gene and suppressed its transcription. Furthermore, LXRs appear to have an important role in arthritis. While early reports diverged as to whether LXRs have a protective function or exacerbate arthritis, recent observations point toward a protective effect of LXRs in arthritis.2 Moreover, LXRs shielded against liver injury in rats induced by intraperitoneal injection of Escherichia coli lipopolysaccharide (LPS) and Staphylococcus aureus peptidoglycan,8 and this effect was partly caused by attenuation of the inflammatory response originating from Kupffer cells. This suggests that LXRs could dampen systemic infections/sepsis, a significant cause of death in surgical intensive-care units today. Activation of LXRs protects against inflammatory reactions in connection with graft vs. host events9 and diabetic nephropathy.10 The protective chronic effects of LXRs on kidney allografts in F344-LEW rats were surprisingly strong and were mediated by LXRα in macrophages. Together this points toward an important role of LXRs as anti-inflammatory regulators in diseases and conditions with an inflammatory component.
Ulcerative colitis and Crohn’s disease represent the two main types of inflammatory bowel diseases (IBDs).11 IBD affects millions of people worldwide, and the incidence of both these ailments are still increasing globally.12 The etiology and underlying molecular mechanisms behind these idiopathic IBDs are currently poorly understood, but involves activation of mucosal/epithelial cells driving an uncontrolled inflammatory response in the small and large intestines.13, 14 In the developing IBD, different immune cells present in IBD lesions produce inflammatory cytokines and chemokines. The epithelial cells also appear to have a substantial role, as they produce and secrete many inflammatory mediators including several chemokines.15, 16, 17 The chemokines induce migration and invasion of additional immune cells including macrophages, neutrophils, and lymphocytes, escalating the inflammatory response. Expression of chemokines is highly upregulated in IBD and correlates with disease activity.15 Current anti-inflammatory treatment of IBD, including inactivation of tumor necrosis factor alpha (TNFα), is inadequate.18 Hence, LXR-mediated inhibition of inflammatory mediators could provide potential novel therapeutic strategies to dampen the symptoms of IBD. To evaluate the potential of LXRs as clinically useful anti-inflammatory targets in IBD, we have investigated the roles of LXR and LXR agonists in colitis. We provide evidence of hitherto unrecognized anti-inflammatory roles of LXRs within the colon.
Results
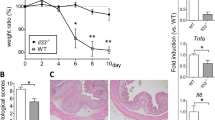
In order to examine the role of LXR in colitis, the effect of dextran sodium sulfate (DSS) in C57Bl/6J (wild type (WT), Lxra−/−, Lxrb−/− and LXRab−/− mice was examined. Both LXRs are expressed in the colon, with mRNA transcripts of Lxra twice as abundant as Lxrb transcripts (Supplementary Figure 1A online). Colitis was induced in mice by administration of DSS in the drinking water. WT mice were pretreated by gavage with GW3965 or vehicle for 4 days, and 2.5% DSS was given to all genotypes as outlined in the Supplementary Figure 1B. WT mice treated with DSS showed an expected loss of body weight starting at day 6, and the weight was reduced by 5% at day 7 and 13% at day 9 (Figure 1a). In comparison, body weight loss at day 7 was more profound in Lxrb−/− mice (14%) and Lxrab−/− mice (15%). At day 7, Lxra−/− mice were less affected with a body weight loss of only 2% (Figure 1a). The effect of DSS on weight reduction was thus significantly lower in Lxrb−/− and Lxrab−/− mice compared with WT and Lxra−/− mice (Figure 1b). No autoregulation of LXRs was observed in the colon from mice treated with the synthetic LXR agonist GW3965 (Supplementary Figure 1A). Administration of GW3965 did not delay the onset of symptoms, but significantly reduced the loss of body weight at day 9 of the experiment (Figure 1a). DSS caused severe symptoms and even death in Lxrb−/− and Lxrab−/− mice. Mice whose disease severity reached the cutoff point defined by the ethical approval for animal research (described in Methods) were killed during the course of the experiment. The cutoff point was documented, and this score indicated that the LXR agonist had a beneficial effect on survival rate, while Lxrab−/− mice showed reduced survival rate compared with WT mice (results not shown).
LXRs protect against dextran sodium sulfate (DSS)- and 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis. Mice were given the LXR agonist GW3965 by gavage (30 mg kg−1 per day) (only in WT mice), and 4 days later 2.5% DSS was mixed in the drinking water and clinical indicators of colitis monitored for 9 (seven for Lxr-deficient mice) days. The clinical indicators measured were (a, b) body weight, (c) colon length, rectal bleeding and diarrhea score. (d) Survival analysis using the Kaplan–Meier method after TNBS injection during the course of the study. For Kaplan–Meier analysis, TNBS-treated mice were compared against control-treated mice (survival curve not presented) (n=7–9 per group). Data are presented as mean±s.e.m. from six individual mice per group, and * and ** indicate P<0.05 and P<0.01, respectively. LXR, liver X receptor; WT, wild type.
The mice were examined for major clinical symptoms associated with colitis during the course of the experiment. Lxrab−/− and Lxrb−/− mice showed an earlier onset of clinical symptoms (results not shown). At the day of sacrifice, all experimental groups receiving DSS showed reduced colon length, increased rectal bleeding, and increased diarrhea, (Figure 1c) and these symptoms were more pronounced in Lxrab−/− and Lxrb−/− mice; mice in these two groups had to be killed 2 days earlier than WT mice. The severe effect of DSS observed in Lxrab−/− mice on colon length is depicted in Supplementary Figure 1C. Gene expression analysis confirmed an induced level of inflammatory markers including interleukin 1 beta (Il1b), Tnfa, and monocyte chemoattractant protein-1 (Mcp1)/Chemokine (C-C motif) ligand 2 (CCL2) in the DSS-treated groups (Supplementary Figure 1D). The expression of these inflammatory genes was equal in Lxrab−/− mice at day 7 compared with WT mice at day 9, suggesting that the inflammatory response is more pronounced at an earlier stage in the Lxrab−/− mice and is in agreement with the earlier onset of clinical symptoms. Next colitis was induced using 2,4,6-trinitrobenzene sulfonic acid (TNBS) that was administered by intra-rectal injections to add a second complementary method to study the protective function of LXRs in colitis. This technique is known to induce a strong and rapid inflammatory response.19 As observed with DSS treatment, the TNBS experiment showed a significantly reduced survival rate of Lxr-deficient mice (Lxrab−/−<Lxrb−/−<Lxra−/−) (Figure 1d). Survival in WT mice was not significantly changed.
The colon from control and DSS-treated mice was prepared for histopathological examination, immunohistological staining of immune cells, and flow cytometry analyses. We observed no structural differences in the colon between the genotypes in the control groups; however, DSS treatment caused severe effects in all genotypes (Supplementary Figure 2A). In agreement with the aforementioned observations, disrupted crypt structure accompanied with increased hyperplasia as well as ulceration was more prominent in the Lxrab−/− and Lxrb−/− mice. DSS caused a marked increase in the accumulation of immune cells (stained dots in the HE panel) and specifically macrophages (F4/80 panel). Infiltration of macrophages was significantly increased in Lxr-deficient mice, particularly in the Lxrab−/− mice at basal conditions (F4/80 panel; enlarged). DSS treatment caused increased infiltration of CD3+ T lymphocytes (Supplementary Figure 2B, upper panel). No infiltration of Pax5+ B lymphocytes was observed (Supplementary Figure 2B, lower panel), indicating that the humoral immune response was not affected. Furthermore, we confirmed that the effects of the DSS treatment were confined to the colon, as no structural changes in the small intestine were observed (Supplementary Figure 2C). CD3+ cells and Pax5+ cells were found in the small intestine, the latter in abundance in Peyer’s plaques, thereby also being a positive control of staining.
Fluorescence-activated cell sorting (FACS) analyses were performed to more accurately quantify the LXR effect on immune cell responses and recruitment. DSS treatment led to increased recruitment of Ly6C+/Ly6G+/CD11b+ immune populations and CD11b+ immune cells (Figure 2a–c), and the activation of LXRs significantly inhibited their recruitment. Next, we addressed the difference of action between the two LXRs by comparing responses in Lxra−/− vs. Lxrb−/− mice. Again DSS treatment led to the increased recruitment of Ly6C+/Ly6G+/b+ immune populations and CD11b+ immune cells in both Lxra−/− and Lxrb−/− mice, and the activation of LXRs led to significantly inhibited recruitment of these immune cell populations in both Lxra−/− and Lxrb−/− mice (Figure 2d–g). This indicates that both LXRs are harboring the potential to suppress the recruitment of these immune cell populations. Using Lxra−/− and Lxrb−/− mice, we next analyzed the T-lymphocyte response. A significant CD3+/CD4+ cell response was observed in the Lxrb−/− mice, but here no significant effect of GW3965 treatment was observed (Figure 2h). Surprisingly, no CD3+/CD4+ cell response was observed in the Lxra−/− mice, suggesting that it might be the acquired T-lymphocyte response that is responsible for the difference observed between the LXR subtypes. Moreover, there are less mononuclear cells in Lxra−/− mice compared with Lxrb−/− mice (Figure 2i).
LXRs regulate dextran sodium sulfate (DSS)-induced immune cell responses. To induce colitis, LXR mice were treated with 2.5% DSS in drinking water. GW3965 (LXR WT) was administrated by gavage as indicated in figure. Colonic lamina propria cells were isolated and analyzed using flow cytometry when the mice reached a 10% weight reduction. (a) Flow cytometry plots of Ly6C/G+ and CD11b+ cells isolated from LXR WT mice. Data are representative of four to five mice (b, c) Fold Ly6C/G+/CD11b+ and CD11b+ immune cell recruitment in LXR WT mice presented as column data with statistics (n=4–5) from flow cytometry experiment presented in a. (d, e) Flow cytometry plots of (d) Ly6C/G+ and CD11b+ cells and (e) CD3+ and CD4+ cells isolated from Lxra−/− and Lxrb−/− mice. Data are representative of four to five mice. (f–h) Fold Ly6C/G+/CD11b+, CD11b+ and CD3+/CD4+ immune cell recruitment in Lxra−/− and Lxrb−/− mice. (i) Total number of mononuclear cells isolated from lamina propria. n=4–5, * and ** indicate P<0.05 and P<0.01, respectively. LXR, liver X receptor; WT, wild type.
The possibility that activation of LXRs could have a positive effect on recovery of DSS-induced colitis was next investigated. Thus, DSS was removed when the mice reached a 10% weight reduction, and the body weight of the mice was monitored for eight consecutive days to observe weight recovery (Figure 3a). Both experimental groups of WT mice continued to lose weight until day 3 (at 20% weight loss) when they started to recover; however, the Lxrab−/− mice continued to lose weight until day 6 (23% weight loss) when they started to recoup. There was a clear trend that mice receiving the GW3965 had faster recovery and this was significant compared with the Lxrab−/− mice. Again, because of ethical considerations, it was necessary to introduce a cutoff point during the time course of this experiment where mice were killed according to our ethical guidelines. This cutoff was reached for 35% (WT), 25% (WT-GW3965), and 43% (Lxrab−/−) of the mice further indicating less recovery of the Lxrab−/− mice and better recovery of the WT-GW3965 mice compared with control mice (Figure 3b). We analyzed gene expression profiles in mice killed at 10% weight reduction (day 0 in Figure 3a) and at the end point at day 8. DSS induced a strong response of known inflammatory genes including Tnfa and interleukine 6 (Il6) as well as chemokines including chemokine (C-X-C motif) ligand 1 (Cxcl1)/keratinocyte chemoattractant (Kc) and Ccl2/Mcp1 (Figure 3c). The inflammatory response was diminished at the end point in parallel with the reversal of weight loss and recovery, and this was more pronounced in mice receiving GW3965.
Activation of LXRs accelerates weight recovery after dextran sodium sulfate (DSS)-induced colitis. Mice were given the LXR agonist GW3965 by gavage (30 mg kg−1 per day) (only the WT mice), and 2.5% DSS was mixed in the drinking water. DSS was withdrawn when the mice reached a 10% weight reduction (set as day 0). Data are presented as mean±s.e.m. from 13 (Lxr WT+DSS), 18 (Lxr WT +DSS/GW), and 8 (Lxrab−/−) individual mice per group. (a) Weight recovery was monitored for the following 8 days. (b) Survival analysis using the Kaplan–Meier method. DSS-treated mice were compared against control-treated mice (survival curve not presented) (n=9 per group). (c) Mice were killed on day 0 or at end point on day 8 in the recovery phase, colon isolated, and mRNA expression profiles of several inflammatory mediators quantified by RT–qPCR. * and ** indicate P<0.05 and P<0.01, respectively. LXR, liver X receptor; WT, wild type.
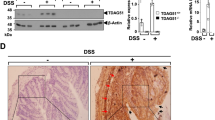
Colonic epithelial cells are known to produce inflammatory factors as well as chemokines attracting immune cells to the site of activation.15 We investigated the potential of LXRs to repress inflammatory responses in the COLO205 and SW480 human colorectal adenocarcinoma cell lines. These cells express both LXRα and LXRβ in equal quantities (Supplementary Figure 3A), and their expression is equivalent to that observed in the murine epithelial cells (data not shown). Cells were pretreated with GW3965 and stimulated with TNFα for 1 and 6 h to induce an inflammatory response. GW3965 caused a 25- to 40-fold induction of expression of the ATP-binding cassette subfamily G member 1 (ABCG1) LXR target gene, indicating a strong effect of the agonist on this cell line (Figure 4a). TNFα induced a robust response of several inflammatory markers including the chemoattractants chemokine (C-X-C motif) ligand 8 (CXCL8)/interleukine 8 (IL8) (mouse homolog to Kc) and chemokine (C-X-C motif) ligand 2 (CXCL2)/macrophage inflammatory protein 2-alpha (MIP2) as well as TNFA, while only a small induction of IL1B was seen at a 6-h treatment. Pretreatment with GW3965 reduced expression of TNFA at both 1 and 6 h, by 90%, expression of IL8 by 50% after 1 h, and expression of CXCL2/MIP2 by 20% at both 1 and 6 h. GW3965 also reduced the TNFα-induced expression of Caspase1, a protease that activates inflammatory processes.20 Chromatin immunoprecipitation revealed that activation of LXRs caused a strong recruitment of LXRs to the LXR-binding element on the ATP-binding cassette subfamily A member 1 (ABCA1) gene promoter (Figure 4b). LXRs were also strongly recruited to the CXCL8/IL8 promoter during inflammatory conditions, but only in the presence of the LXR agonist. The anti-inflammatory effect of GW3965 was confirmed using an additional human colorectal adenocarcinoma cell line, the SW480. The inflammatory response in this cell line was induced by both LPS and TNFα and co-treatment with GW3965 leads to significant repression at 1-, 4-, and 24-h incubation (Supplementary Figure 3B), indicating that the LXR effect was not limited to a specific cell line. Moreover, the natural LXR agonist 22(R)-hydroxycholesterol (22-H) was equally efficient to repress expression of TNFA (Figure 4c). When LXRB expression was reduced by a specific siRNA in the SW480 cell line (Figure 4d), there was no effect on induction of CXCL8/IL8 and TNFA; however, repression by GW3965 was abolished (Figure 4e), suggesting LXRβ to be the dominant subtype repressing the inflammatory response in SW480 cells.
Suppression of inflammatory mediators in human colonic epithelial cells is mediated by LXRβ. The Colo205 (a, b) or SW480 cells (c–e) were pretreated with vehicle (DMSO) or 5 μM GW3965 for 16 h followed by stimulation with TNFα or LPS for 1- or 6 h as indicated. Selected genes were quantified by RT–qPCR. (a) mRNA was isolated from TNFα-treated COLO205 cells and expression quantified. (b) Association of LXRs with their cognate binding site in ABCA1 or in the gene promoter region of CXCL8/IL8 after 1 h stimulation with TNFα in pretreated cells was analyzed by ChIP assays. (c) mRNA was isolated from LPS-treated SW480 using both the synthetic (GW3965) and the endogenous (22(R)-hydroxycholesterol) LXR agonists. mRNA was isolated and the expression profiles of TNFA were quantified. (d) SW480 cells were treated with siLuciferase or specific siRNA targeting LXRB. LXRB expression both at the mRNA and protein levels was quantified. (e) SW480 cells were treated with siLuciferase or specific siRNA targeting LXRB±LPS/GW3965 and expression profiles of TNFA and CXCL8/IL8 quantified. Error bars are represented as mean±s.e.m. * and ** indicate P<0.05 and P<0.01, respectively. ChIP, chromatin immunoprecipitation; LPS, lipopolysaccharide; LXR, liver X receptor; TNFα, tumor necrosis factor-α.
The observations using the colonic cell lines encouraged us to investigate whether similar effects took place in vivo in the colon of mice. For this purpose, we used intraperitoneal injection of LPS that activates the toll-like receptor 4 (TLR4) pathway. LPS also yields a milder immune response than DSS or TNBS, and it produces a more equal inflammatory response within the groups than DSS or TNBS. WT and Lxr-deficient mice were given LPS for 1 h±GW3965 (except the LXRab−/− mice) and the effect on mRNA expression of inflammatory genes analyzed in the colon. Figure 5a shows that GW3965 represses induction of several inflammatory and chemokine genes including Tnfa, Cxcl1/Kc, Cxcl2/Mip2, Ccl2/Mcp1, and Il17a. The repression was significant for Tnfa and Il17a in WT mice and significant for all the genes in the Lxra−/− mice. The LXR effects were confirmed for TNFα at the protein level (Figure 5b). The gene expression profiles and repression were confirmed for Tnfa and Cxcl1/Kc, but not for Cxcl2/Mip2 and Ccl2/Mcp1, in isolated epithelial cells collected from the colon (Figure 5d), suggesting that the LXR effect takes place both in colonic epithelial cells and in resident and infiltrating immune cells in the colon. Interestingly, the expression of Lxrb was induced threefold in Lxra−/− mice (Figure 5c). This might explain why the repression is stronger in Lxra−/− mice compared with WT mice. It further suggests that LXRα could have a slightly different role by dampening the effect of LXRα on this pathway, a concept we have reported on earlier.21
Activation of LXRβ suppresses inflammatory mediators in the colon and colonic epithelial cells. Mice were pretreated with the LXR agonist GW3965 by gavage (30 mg kg−1 per day) (not Lxrab−/− mice) for 16 h and given intraperitoneal injections of LPS for 1 h. Data are presented as mean±s.e.m. from six individual mice per group. (a) The colon was isolated, and mRNA expression profiles of several inflammatory mediators quantified by RT–qPCR. (b) The colon was isolated and incubated±GW3965 for 16 h. Medium was collected and analyzed for protein levels of TNFα by ELISA. (c) The colon was isolated and mRNA expression profiles of Lxrb quantified by RT–qPCR. (d) The colon was isolated, colonic epithelial cells collected, and mRNA expression profiles of several inflammatory mediators quantified by RT–qPCR. * and ** indicate P<0.05 and P<0.01, respectively. ELISA, enzyme-linked immunosorbent assay; LPS, lipopolysaccharide; LXR, liver X receptor; TNFα, tumor necrosis factor-α.
Next, we wanted to confirm the anti-inflammatory effects of LXRs in vivo in the colon and to investigate the response in isolated immune cell populations. LPS induced recruitment of Ly6C+/Ly6G+/CD11b+ cells, and this recruitment was significantly higher in Lxrab−/− mice compared with WT and single LXR-deficient mice (Figure 6a and b). The difference between WT and Lxrab−/− mice was particularly strong for the CD11b+ immune population (Figure 6c). We further purified CD11b+ immune cells to analyze the LXR effect on inflammatory responses within the immune cells. The expression levels of LXRα and LXRβ are 10- and 5-fold higher, respectively, in CD11b+ cells compared with colon epithelial cells (Supplementary Figure 3D). We observed that GW3965 significantly suppressed LPS-induced expression of IL-6 and IL-1β, while a strong trend (P=0.051) was observed for TNFα (Figure 6d). However, the chemoattractants including KC and MCP-1 were not affected by GW3965, indicating that the effect on chemoattractants is limited to the colonic epithelial cells. The effect of a single dose of an inflammatory stimulus is transient both in colorectal adenocarcinoma cell line (Supplementary Figure 3B) and in vivo (Supplementary Figure 3C). We asked whether a prolonged inflammatory response could be observed in Lxr-deficient mice in addition to an exaggerate response. Indeed, in Lxrab−/− mice there was a substantially higher level of inflammatory markers in the colon 24 h after LPS treatment than in WT mice (Figure 6e), supporting the slower recovery of these mice showed (Figure 3a).
Lxr-deficient mice have a prolonged state of upregulated inflammatory mediators. To induce inflammation, mice were given intraperitoneal injections of LPS for 24 h and mice receiving the LXR agonist GW3965 (d) were pretreated (16 h) by gavage (30 mg kg−1). (a) Flow cytometry dot plots of lamina propria Ly6C/G+ and CD11b+ cells isolated from the colon. Data are representative of four to five mice. (b, c) Column data with statistics (n=4–5) from flow cytometry experiment presented in a. (d) Lamina propria mononuclear immune cells from LXR WT mice treated as indicated in figure were purified and CD11b+ cells isolated. mRNA expression profiles of inflammatory mediators in CD11b+ cells quantified by RT-qPCR. Data are presented as mean±s.e.m. from four individual mice per group. (e) The colon was isolated and mRNA expression profiles of several inflammatory mediators quantified by RT-qPCR. Data are presented as mean±s.e.m. from eight individual mice per group. * and ** indicate P<0.05 and P<0.01, respectively. LPS, lipopolysaccharide; LXR, liver X receptor; WT, wild type.
The metabolic effects of LXRs in the colon have never been investigated, but the known metabolic roles of LXRs in several other tissues raise the question whether hitherto unknown effects of LXRs on colon metabolism could have a role in the LXR-induced regulation of immune responses. We analyzed the RNA expression profiles of several important genes involved in lipid and cholesterol metabolism in the colon and in colon epithelial cells. We confirmed that most of these metabolic genes also are LXR target genes in the colon (Supplementary Figure 4A and B). However, we could not associate any of the changes in expression levels, in relation to GW3965 response or between the LXR knockout mice, with the phenotypes observed in modulating immune responses. Thus, we conclude that the effects on metabolic pathways in the colon are separated from the effects on the inflammatory pathways. Nevertheless, the loss of LXRβ led to significantly reduced or completely abolished responses to GW3965, further supporting the important role of LXRβ in the colon.
Four independent studies where genome-wide mRNA expression levels are reported were analyzed for the expression of LXRA and LXRB. Expression levels of both LXRA (P∼1 × 10−5) and LXRB (P∼1 × 10−3) are significantly downregulated in the inflamed colon from both Crohn’s disease and ulcerative colitis compared with the non-inflamed colon (Figure 7 and Supplementary Figure 5), suggesting that reduced levels of LXRs is part of the IBD pathology.
Expression of LXRs are suppressed in IBD. The mRNA expression levels of (a) LXRA (NR1H3) and (b) LXRB (NR1H2) were extracted from four independent studies where human colon samples had been collected from non-inflammatory (N; n=28), Crohn’s disease (CD; n=101), and Ulcerative colitis (UC; n=45). Mean expression levels from the four combined studies are presented as well as the combined expression levels of CD and UC, indicated as IBD, compared to non-inflammatory colon. All comparisons have a statistical significance of at least P<0.01. IBD, inflammatory bowel disease; LXR, liver X receptor.
Discussion
Using two different inducers of colitis in mice, DSS and TNBS, as well as LPS treatment, we show that loss of LXR, in particular of LXRβ, leads to higher susceptibility to colitis and worsening of colitis-related symptoms in the colon. We observe that the activation of LXRs protects against colitis and reduces inflammation, while loss of LXRs has an opposite effect. When colitis was induced in Lxr-deficient mice, they showed a marked worsening of clinical symptoms including ulcers, diarrhea and rectal bleeding, and increased mortality in both colitis DSS- and TNBS colitis models. Loss of LXRs led to the increased invasion of macrophage and increased as well as a prolonged release of inflammatory mediators in the colon. Treatment with an LXR agonist in colitis-induced mice showed improved recovery, enhanced survival, and suppressed expression of inflammatory mediators—both in vivo and in human colon cell lines. Finally, loss of LXRβ contributed to an exacerbated acquired immune response. Thus, the protective role of LXRs in the development of colitis appears to represent a general mechanism, evident in both the innate and adaptive immune response.
The suppressive effect of LXRs on several inflammatory markers in IBD is interesting. The most effective and used therapy towards IBD today is aimed at blocking the TNFα signaling using TNFα antibodies.18 Furthermore, recent evidence indicates that IL17A, the cytokine produced by TH-17 lymphocytes, is a major factor driving an exaggerated immune response in the IBD.22 We show that loss of LXRs and activation of LXRs lead to induced and suppressed levels of IL17A, respectively. Together with TNFα, IL17A is a potent inducer of chemokine production in colonic epithelial cells—particularly CXCL1/KC and CXCL8/IL8.17, 23 This further supports the concept that LXRs modulate immune responses associated with colitis and that the effect of LXR on TNFα and IL17A is part of the regulatory pathway of LXR-related chemokine production.
More than 40 chemokines have been identified so far, and they are responsible for adhesion and homing of immune cells to the site of inflammation.15 We show that loss of LXRs induced expression of chemokines, whereas activation of LXRs suppressed expression of chemokines including CXCL1/KC, CXCL2/MIP2, CXCL8/IL8, CCL2/MCP1, and CXCL10. It has been known for a long time that patients suffering from IBD have increased levels of CXCL8/IL8 in the colon.24, 25, 26 CXCL8/IL8 is produced by immune cells and epithelial cells at the active site of colitis.27 Several studies have shown high levels of CXCL10,28 CCL2,29, 30 and CXCL131 in colitis, and it was observed that loss of CXCL1032 or CCL233 reduced the severity of DSS-induced colitis in mice. These chemokines have the property of recruiting several types of immune cells including neutrophils, macrophages, and T-lymphocytes, thereby exacerbating the immune response. By suppressing both inflammatory cytokines and chemokines in immune cells and colonic epithelial cells, LXRs affect the immunopathogenesis of IBD at multiple levels. The observations made in the chromatin immunoprecipitation assays indicate that in an inflammatory environment the activation of LXRs leads to their recruitment to inflammatory genes, thereby reducing the transcriptional rate of these genes.
It appears that the LXR subtypes have both common and exclusive roles in the immune system. While LXRβ has been reported to be responsible for several aspects of repression,34, 35, 36 LXRα seems more important in macrophages37 and dendritic cells.38, 39 In addition, it was reported that post-translational modifications underlying molecular mechanisms of repression were connected to different protein assembly networks.34, 40 Our observations point toward a more pronounced protective role of LXRβ than LXRα in both DSS-, TNBS- and LPS-induced colitis and inflammation. We show that both LXR subtypes are relatively equally expressed in the colon of mice, suggesting that the more marked effects observed by LXRβ are not due to differences in the expression of the two LXRs. The role of LXRα could be more restricted to the innate immune response via macrophages and dendritic cells, while LXRβ could be responsible for the immune response in colonic epithelial cells and the acquired immune response via CD3+/CD4+ lymphocytes. The increased levels of inflammatory cytokines and chemokines from epithelial cells have a very important part in escalating the immune response in IBD, and this might explain the severity of the disease symptoms observed in the absence of LXRβ. Also, there is a threefold increased expression of Lxrb in Lxra-deficient mice, which might explain why Lxra-deficient mice have milder symptoms than Lxrb-deficient mice.
The adaptive immune response, initiated by dendritic cells presenting antigens to naive T cells in secondary lymphoid organs (Peyer’s patches and mesenteric lymph nodes), is an important part of the inflammatory response in IBD.41 These activated T cells circulate to the intestinal lamina propria, where they carry out effector functions including modulation of cytokine profiles adding to the severity of the exaggerated immune response in IBD. Thus, the lower level of CD3+/CD4+ cells observed in LXRα−/− mice upon DSS-induced inflammation is interesting, as it implies that there is a specific role of LXRα in regulating T-cell responses, which demands further detailed investigations to uncover the exact mechanisms underlying this observation.
It was recently reported that genetic variations in several nuclear receptors including LXRB are associated with Crohn’s disease and ulcerative colitis42 further implying a role of LXRβ. We show that expression of both LXRA and LXRB is suppressed in the inflamed colon from subjects with IBD. Lower expression of the LXR would suggest reduced anti-inflammatory potential and tuning down LXR signaling might well be a mechanism that exaggerates the pathology of IBD.
As the development of new and efficient therapies for IBD has proven to be difficult,18 new targets are needed. Drugs targeting LXRs in the whole body have unwanted side effects including increased production of triglycerides.1 However, development of subtype- or tissue-specific LXR modulators continues to be the goal of the pharmaceutical industry. There has been one report of an intestine-specific LXR agonist.43 If better LXR agonists can be made with fewer harmful side effects, they might well represent novel strategies to prevent and treat IBD. Here we show that the natural LXR agonist 22(R)-hydroxycholesterol (22-H) could repress expression of Tnfa. Interestingly, phytosterols, the equivalent of mammalian cholesterol in plants, are activators of LXRs44 as are a plethora of compounds from microorganisms and plants.45 In addition to 22(R)-hydroxycholesterol, a subset of endogenous oxysterol LXR agonists appears to trigger the repression of the inflammatory pathway.4 Hence, it is possible that dietary phytosterols and/or synthetic compounds that activate LXRs could have a multilevel effect in prevention or treatment of IBD.
Methods
Animal studies. Experimental protocols were approved by Karolinska Institutet Animal Care and Use Committee. The Lxr-deficient mouse models have been described.46, 47 The DSS effect in Lxrb−/− and Lxrab−/− mice caused severe symptoms and even death. The experiments including all Lxr-deficient mice were therefore terminated at day 7 due to ethical considerations. Mice that reached a defined score of points (cutoff point) based on their general well-being according to our ethical approval for animal research (% weight loss, rectal bleeding, general behavioral, and general activity level) were killed during the course of the experiment. The cutoff point was documented and reported as survival rate. C57Bl/6J (females, 10-weeks old) were fed a chow diet and pretreated for 4 days with GW3965 (30 mg kg−1 per day) or vehicle by gavage. Colitis was induced by adding 2.5% (w/v) DSS (Dextran Sulfate Sodium, TdB Consultancy AB, Uppsala, Sweden) to the drinking water for 7 (Lxra−/−, Lxrb−/− and Lxrab−/−) and 9 (C57BI/6J (wild type (WT)) days. To study weight recovery after DSS-induced colitis, C57Bl/6J and Lxrab−/− mice were given 2.5% DSS in the drinking water and DSS was withdrawn upon a weight reduction of ∼10%. Colitis was also induced in mice using 2.5% (w/v) TNBS (Sigma-Aldrich, St Louis, MO) in 50% ethanol. The mice were lightly anesthetized in a glass chamber using isoflurane (Baxter Medical AB, Stockholm, Sweden), and 100 μl TNBS solution was injected into the rectum (∼3 cm) using a round tipped syringe. To distribute the TNBS, the mice were hold in a vertical position for 1 min after the injection. To induce inflammation in mice, intraperitoneal injections (10 mg kg−1) of LPS (Sigma-Aldrich) were given for 1 h±GW3965 (pretreated for 24 h) or for 24 h without GW3965. In all experiments, mice were sedated by isofluran following cervical dislocation, and organs were quickly removed and frozen in liquid nitrogen. Rectal bleeding and diarrhea were monitored by manual analyses of the level of blood and water content in the feces. Three persons independently performed these analyses. It was recently shown that LXRβ−/− mice have slightly increased water intake48 compared with WT mice; however, no difference in water intake between LXRα−/− and LXRβ−/− mice (where large differences in inflammatory phenotypes are seen) was observed. In addition, the results from TNBS and LPS treatments as well as from cell culture experiments further indicate that the inflammatory phenotypes are linked to LXRβ. Thus, we do not believe that the observations in the DSS experiments were solely due to increased water intake in mice lacking LXRβ.
Enzyme-linked immunosorbent assay. For TNFα protein levels, mice were treated for 1 h with LPS±GW3965 (pretreated for 24 h) and the colon removed, opened longitudinally, and washed three times with PBS containing gentamycin (20 μg ml−1). Biopsies were moved to 24-well plates containing 500 μl RPMI1640 culture medium and incubated for 16 h at 37 °C in a cell culture incubator and supernatant collected. Protein levels of TNFα were measured using the mouse ELISA kit (R&D Systems, Minneapolis, MN) according to instructions provided by the manufacturer.
Isolation of immune cells and flow cytometry. Isolation of lamina propria immune cells from the colon was performed essentially as previously described.49 In brief, the colon was removed, opened longitudinally, and cropped into 1 cm pieces. The pieces were incubated twice in predigestion solution (HBSS, 5 mM EDTA and 1 mM dithiothreitol) for 20 min at 37 °C under slow rotation and passed through a 100-μm cell strainer. The pieces were washed in 1 × PBS and cropped to 1 mm pieces and incubated in digestion solution (100 ml PBS, 50 mg collagenase D, 50 mg DNase I and 300 mg dispase II) for 20 min at 37 °C under slow rotation. The cell solution was vortexed and passed through a 40-μm cell strainer and collected in fresh digestion solution. This was repeated until the connective tissue was absent in the cell strainer. The flow through was centrifuged at 500 g for 10 min and pellet resuspended in cold FACS buffer. The cells were once again centrifuged at 500 g for 10 min and pellet resuspended in 10 ml percoll (40%). The cell suspension was carefully overlaid on top of 80% percoll and centrifuged for 20 min at 1,000 g and the interphase was isolated using a Pasteur pipette. The isolated cells were incubated with AlexaFluor488-CD11b, clone M1/70 (BD Biosciences); PE-Ly6G/6C, clone RB6-8C5 (BD Biosciences); PerCP-CD3, clone 145-2C11 (BD Biosciences); and APC-CD4, cloneRM4-5 (BD Biosciences) antibodies and measured by flow cytometry using a FACS calibur (BD, Franklin Lakes, NJ) flow cytometer, and data analysis was performed using cell quest pro software. To subtract necrotic cells from the flow cytometry analysis, the cell population was stained with propidium iodide. To analyze mRNA expression in CD11b+ cells, Easycoll isolated mononuclear immune cells were further purified using CD11b-pluribeads (PluriSelect, Leipzig, Germany) for positive isolation of CD11b+ cells.
Histopathology and immunohistochemistry. Colons were flushed and fixed in 4% paraformaldehyde. After 24 h, the paraformaldehyde was exchanged with 70% ethanol for 24 h after which the tissues were embedded in paraffin. After sectioning, slides were stained with hematoxylin and eosin according to the standard protocol. Immunohistology was performed according to the standard protocols and antibody staining as follows: F4/80 (AbD Serotec, Oxford, UK) rat anti-mouse F4/80 antigen (MCAP497), diluted 1:100; antigen retrieval, digest-All 2 (Zymed, Invitrogen, Carlsbad, CA, 00-3008); secondary, rabbit anti-rat biotinylated (Dako, Agilent Technologies, Glostrup, Denmark, E4068), diluted 1:400; Pax5 (C-20, Santa Cruz Biotechnology Inc., Santa Cruz, CA, sc-1974) goat polyclonal, diluted 1:2500; antigen retrieval: DIVAdecloaker (Biocare Medical DV2004MX, Concord, CA); Secondary, rabbit anti-goat biotinylated (Dako, E0466), diluted 1:300; CD3 (cloneSP7, Labvision, RM-9107-S0, Stockholm, Sweden), diluted 1:200; antigen retrieval, DIVAdecloaker (Biocare Medical DV2004MX); secondary, goat anti rabbit biotinylated (Dako, E0432), diluted 1:300.
Expression analysis from human colon samples. The expression patterns of LXRA and LXRB were studied in four re-analyzed and computationally integrated gene expression Affymetrix GeneChip experiments (public IDs: GSE10616 (pmid: 18758464), GSE16879 (pmid: 19956723), GSE9686 (pmid: 18069684), and MEXP2083 (pmid: 21830273)), where colon biopsies from IBD patients and matched healthy individuals were assayed. Raw microarray data files (.CEL files) were retrieved from the public databases NCBI GEO (pmid: 23193258) and EBI ArrayExpress (pmid: 23193272), imported into R v.2.15, and analyzed with the BioConductor facilities (pmid: 15461798). Because of known flaws in the Affymetrix original probe design (pmid: 16284200), a custom CDF file v.14 was retrieved from the BrainArray website (http://brainarray.mbni.med.umich.edu), where only the probes uniquely matching the Entrez Gene IDs (v. Mar-10-2011) were re-arranged into newly defined probe sets. The raw data were then preprocessed (background corrected, normalized with the quantile method and summarized with the median polish method) with the RMA algorithm (pmid: 12925520). The data from the four experiments were finally integrated eliminating the batch effect with the empirical Bayes-based algorithm ComBat (pmid: 16632515). Differential expression was assessed with linear models followed by the empirical Bayes method (pmid: 16646809).
Statistical analysis. Data are expressed as mean±s.e.m. and analyzed by two-tailed t-test or by one-way analysis of variance (ANOVA) with Tukey’s test for multiple comparisons. For survival analysis, the Kaplan–Meier method was used and the survival compared with log-rank and Gehan-Wilcoxon tests. In the survival curves, the error bars indicate 95% confidence interval.
References
Jakobsson, T., Treuter, E., Gustafsson, J.A. & Steffensen, K.R. Liver X receptor biology and pharmacology: new pathways, challenges and opportunities. Trends Pharmacol. Sci. 33, 394–404 (2012).
Steffensen, K.R., Jakobsson, T. & Gustafsson, J.A. Targeting liver X receptors in inflammation. Expert opin. Therapeut. Targets 17, 977–990 (2013).
Joseph, S.B., Castrillo, A., Laffitte, B.A., Mangelsdorf, D.J. & Tontonoz, P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat. Med. 9, 213–219 (2003).
Ghisletti, S. et al. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARgamma. Mol. Cell 25, 57–70 (2007).
Hindinger, C. et al. Liver X receptor activation decreases the severity of experimental autoimmune encephalomyelitis. J. Neurosci. Res. 84, 1225–1234 (2006).
Xu, J., Wagoner, G., Douglas, J.C. & Drew, P.D. Liver X receptor agonist regulation of Th17 lymphocyte function in autoimmunity. J. Leukoc. Biol. 86, 401–409 (2009).
Cui, G. et al. Liver X receptor (LXR) mediates negative regulation of mouse and human Th17 differentiation. J. Clin. Invest. 121, 658–670.
Wang, Y.Y. et al. Liver X receptor agonist GW3965 dose-dependently regulates LPSmediated liver injury and modulates post-transcriptional TNFalpha production and p38 MAPK activation in liver macrophages. Shock 32, 548–553 (2009).
Kiss, E. et al. Suppression of chronic damage in renal allografts by Liver X receptor (LXR) activation relevant contribution of macrophage LXRalpha. Am. J. Pathol. 179, 92–103 (2011).
Kiss, E. et al. Lipid droplet accumulation is associated with an increase in hyperglycemia-induced renal damage: prevention by Liver X Receptors. Am. J Pathol. 182, 727–741 (2013).
Loftus, E.V. Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology 126, 1504–1517 (2004).
Molodecky, N.A. et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142, 46–54 e42; quiz e30 (2012).
Xavier, R.J. & Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448, 427–434 (2007).
Strober, W., Fuss, I. & Mannon, P. The fundamental basis of inflammatory bowel disease. J. Clin. Invest. 117, 514–521 (2007).
Atreya, R. & Neurath, M.F. Chemokines in inflammatory bowel diseases. Dig. Dis. 28, 386–394 (2010).
Lazennec, G. & Richmond, A. Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol. Med. 16, 133–144 (2010).
Lee, J.W. et al. Differential regulation of chemokines by IL-17 in colonic epithelial cells. J. Immunol. 181, 6536–6545 (2008).
Macdonald, T.T. Inside the microbial and immune labyrinth: totally gutted. Nat. Med. 16, 1194–1195.
Wirtz, S., Neufert, C., Weigmann, B. & Neurath, M.F. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2, 541–546 (2007).
Mariathasan, S. et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430, 213–218 (2004).
Hu, X. et al. LXRbeta activation increases intestinal cholesterol absorption, leading to an atherogenic lipoprotein profile. J Intern. Med. 272, 452–464 (2012).
Rovedatti, L. et al. Differential regulation of interleukin 17 and interferon gamma production in inflammatory bowel disease. Gut 58, 1629–1636 (2009).
Awane, M., Andres, P.G., Li, D.J. & Reinecker, H.C. NF-kappa B-inducing kinase is a common mediator of IL-17-, TNF-alpha-, and IL-1 beta-induced chemokine promoter activation in intestinal epithelial cells. J. Immunol. 162, 5337–5344 (1999).
Mahida, Y.R. et al. Enhanced synthesis of neutrophil-activating peptide-1/interleukin-8 in active ulcerative colitis. Clin. Sci. 82, 273–275 (1992).
Izzo, R.S. et al. Neutrophil-activating peptide (interleukin-8) in colonic mucosa from patients with Crohn's disease. Scand. J. Gastroenterol. 28, 296–300 (1993).
Raab, Y., Gerdin, B., Ahlstedt, S. & Hallgren, R. Neutrophil mucosal involvement is accompanied by enhanced local production of interleukin-8 in ulcerative colitis. Gut 34, 1203–1206 (1993).
Banks, C., Bateman, A., Payne, R., Johnson, P. & Sheron, N. Chemokine expression in IBD. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn’s disease. J. Pathol. 199, 28–35 (2003).
Uguccioni, M. et al. Increased expression of IP-10, IL-8, MCP-1, and MCP-3 in ulcerative colitis. Am. J. Pathol. 155, 331–336 (1999).
Mazzucchelli, L. et al. Differential in situ expression of the genes encoding the chemokines MCP-1 and RANTES in human inflammatory bowel disease. J. Pathol. 178, 201–206 (1996).
Reinecker, H.C. et al. Monocyte-chemoattractant protein 1 gene expression in intestinal epithelial cells and inflammatory bowel disease mucosa. Gastroenterology 108, 40–50 (1995).
Egesten, A. et al. The proinflammatory CXC-chemokines GRO-alpha/CXCL1 and MIG/CXCL9 are concomitantly expressed in ulcerative colitis and decrease during treatment with topical corticosteroids. Int. J. Colorectal Dis. 22, 1421–1427 (2007).
Sasaki, S. et al. Blockade of CXCL10 protects mice from acute colitis and enhances crypt cell survival. Eur. J. Immunol. 32, 3197–3205 (2002).
Andres, P.G. et al. Mice with a selective deletion of the CC chemokine receptors 5 or 2 are protected from dextran sodium sulfate-mediated colitis: lack of CC chemokine receptor 5 expression results in a NK1.1+ lymphocyte-associated Th2-type immune response in the intestine. J. Immunol. 164, 6303–6312 (2000).
Venteclef, N. et al. GPS2-dependent corepressor/SUMO pathways govern anti-inflammatory actions of LRH-1 and LXRbeta in the hepatic acute phase response. Genes Dev. 24, 381–395.
Bensinger, S.J. et al. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell 134, 97–111 (2008).
Huang, W. et al. Coronin 2A mediates actin-dependent de-repression of inflammatory response genes. Nature 470, 414–418.
Joseph, S.B. et al. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell 119, 299–309 (2004).
Geyeregger, R. et al. Liver X receptors regulate dendritic cell phenotype and function through blocked induction of the actin-bundling protein fascin. Blood 109, 4288–4295 (2007).
Torocsik, D. et al. Activation of liver X receptor sensitizes human dendritic cells to inflammatory stimuli. J. Immunol. 184, 5456–5465.
Lee, J.H. et al. Differential SUMOylation of LXRalpha and LXRbeta mediates transrepression of STAT1 inflammatory signaling in IFN-gamma-stimulated brain astrocytes. Mol. Cell 35, 806–817 (2009).
Abraham, C. & Cho, J.H. Inflammatory bowel disease. N. Engl J. Med. 361, 2066–2078 (2009).
Andersen, V. et al. Polymorphisms in NF-kappaB, PXR, LXR, PPARgamma and risk of inflammatory bowel disease. World J. Gastroenterol. 17, 197–206 (2011).
Yasuda, T. et al. Tissue-specific liver X receptor activation promotes macrophage reverse cholesterol transport in vivo. Arterioscler. Thromb. Vasc. Biol. 30, 781–786 (2010).
Plat, J., Nichols, J.A. & Mensink, R.P. Plant sterols and stanols: effects on mixed micellar composition and LXR (target gene) activation. J. Lipid Res. 46, 2468–2476 (2005).
Viennois, E. et al. Selective liver X receptor modulators (SLiMs): What use in human health? Mol. Cell. Endocrinol. 351, 129–141 (2011).
Jakobsson, T. et al. GPS2 is required for cholesterol efflux by triggering histone demethylation, LXR recruitment, and coregulator assembly at the ABCG1 locus. Mol. Cell 34, 510–518 (2009).
Alberti, S. et al. Hepatic cholesterol metabolism and resistance to dietary cholesterol in LXRbeta-deficient mice. J. Clin. Invest. 107, 565–573 (2001).
Gabbi, C. et al. Central diabetes insipidus associated with impaired renal aquaporin-1 expression in mice lacking liver X receptor beta. Proc. Natl Acad. Sci. USA 109, 3030–3034 (2012).
Weigmann, B. et al. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat. Protoc. 2, 2307–2311 (2007).
Acknowledgements
K.R.S. holds a research assistant professorship from the Swedish Research Council (522-2008-3745). This study was supported by the Karo Bio Research Foundation (K.R.S.), Swedish Research Council (M.D’A., J.-Å.G. and E.T.), the Swedish Cancer Society (E.T.), the Robert A. Welch Foundation (E-0004, J.-Å.G.), the Texas Emerging Technology Fund under Agreement 18 no. 300-9-1958 (J.-Å.G.), the Center for Biosciences (J.-Å.G. and E.T.), and the Novo Nordisk Foundation (E.T.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Author contributions
K.R.S., T.J. and E.T. designed the study and the data were analyzed by K.R.S. and T.J. K.R.S. and T.J. performed most of the experiments with contributions of N.V., L.-L.V. and T.H. N.V. performed in vivo studies, L.-L.V. performed immunohistochemistry and T.H. performed cell culture assays. M.D’A. and D.G. performed bioinformatic expression analysis of human samples, and K.R.S. and T.J. wrote the manuscript with contributions from E.T. and J.-Å.G.
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Supplementary information
Rights and permissions
About this article
Cite this article
Jakobsson, T., Vedin, LL., Hassan, T. et al. The oxysterol receptor LXRβ protects against DSS- and TNBS-induced colitis in mice. Mucosal Immunol 7, 1416–1428 (2014). https://doi.org/10.1038/mi.2014.31
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2014.31
This article is cited by
-
Electroacupuncture inhibits visceral pain via adenosine receptors in mice with inflammatory bowel disease
Purinergic Signalling (2019)
-
Liver X receptors regulate hepatic F4/80 + CD11b+ Kupffer cells/macrophages and innate immune responses in mice
Scientific Reports (2018)
-
Molecular and functional heterogeneity of IL-10-producing CD4+ T cells
Nature Communications (2018)
-
Rifaximin is an effective alternative to metronidazole for the treatment of chronic enteropathy in dogs: a randomised trial
BMC Veterinary Research (2016)