Abstract
The probiotic Escherichia coli Nissle 1917 (EcN) is widely used to maintain remission in ulcerative colitis. This is thought to be mediated by various immunomodulatory and barrier-stabilizing effects in the intestine. In this study, the mechanisms of barrier modulation by EcN were studied in the human epithelial HT-29/B6 cell culture model.
EcN supernatant increased transepithelial resistance (TER) and reduced permeability to mannitol because of sealing of the paracellular passage pathway as revealed by two-path impedance spectroscopy. This increase in TER was attributed to the TcpC protein of EcN. TcpC induced protein kinase C-ζ (PKCζ) and extracellular-signal-regulated kinase 1/2 (ERK1/2) phosphorylation, which in turn resulted in upregulation of the barrier-forming tight junction protein claudin-14. By specific silencing of protein expression by small interfering RNA (siRNA), the sealing function of claudin-14 was confirmed. In conclusion, the TcpC protein of EcN affects innate immunity by improving intestinal barrier function through upregulation of claudin-14 via PKCζ and ERK1/2 signaling.
Similar content being viewed by others
INTRODUCTION
The probiotic bacterium Escherichia coli Nissle 1917 (EcN, Mutaflor) has been applied for many years in the therapy of gastrointestinal disorders. Clinical studies confirmed its beneficial effects in patients with inflammatory bowel disease, for example, to prevent relapse in ulcerative colitis.1, 2 Consequently, this microorganism was subject to intense research in the past decades and different beneficial properties were reported. Although EcN is not capable of penetrating into mucosal cells by itself, it was found to inhibit invasion of pathogenic enteroinvasive microorganisms3 that are often causative agents of diarrhea and inflammation. In addition, EcN comprises immune modulatory properties, for example, it induced expression of the antimicrobial peptide β-defensin-2 in human colonic epithelial cells.4 This was mediated by flagellin that is present in EcN culture supernatant.5 Moreover, a second bacterial factor TcpC was reported to play a role for protecting mice from dextran sodium sulfate-induced colitis (Julia Frick, University Tübingen, personal communication). Furthermore, EcN was suggested to directly stabilize the intestinal barrier, a main structural feature of innate immunity in the gut, by regulating barrier-associated proteins in epithelial cells. Accordingly, it was described that EcN stimulated the expression of zonula occludens protein-2 (ZO-2) and desmosome protein pinin in T84 cells.6, 7 Also, expression of zonula occludens protein-1 (ZO-1) was reported to be enhanced by EcN administration to germfree mice as well as in a mouse model of colitis.8
Epithelial tight junctions (TJs) localized in the apical contact zone of adjacent enterocytes play a key role in regulating intestinal barrier properties.9, 10 ZO proteins (ZO-1, -2, and -3) are membrane-associated cytosolic proteins functioning as scaffold proteins in the organization of TJ strands11, 12, 13 by forming an anchor for different types of transmembranal proteins, such as occludin,14 claudins,15 and tricellulin.16 The latter are strand-forming TJ proteins known to control paracellular diffusion. In recent years, several claudins were linked either with a sealing function or a specific channel function. Hence, the tissue-specific structural and physiological function of the TJs depends on the composition of these TJ proteins and their interaction with each other as well as with scaffold proteins, cytoskeleton-associated proteins, and regulatory components.17 The integrity of the TJs can be influenced by different intra- or extracellular stimuli. In cell culture studies this was mediated by associated protein kinases, such as the mitogen-activated protein kinases,18 protein kinase C (PKC),19 or phosphatidylinositol-3-kinase.20
Several studies suggested probiotic microorganisms as regulators of TJs and thus of the intestinal epithelial barrier. Recently, a factor secreted by Bifidobacterium infantis was shown to enhance occludin and ZO-1 protein level and to decrease the expression of the channel-former claudin-2 in T84 cells.21 In mice, the probiotic mixture VSL3 prevented colitis-associated changes in occludin, ZO-1, and claudin-1, -3, -4, and -5.22
However, so far, the impact of EcN on proteins that directly determine properties of the paracellular epithelial barrier—the sealing or channel-forming TJ proteins—has not been described. Therefore, this study addressed the mechanisms of EcN-mediated increase of paracellular barrier function with special attention to claudins and tricellulin. In contrast to other studies,3, 8 we investigated unchallenged epithelial cells treated with a highly active EcN culture supernatant.
RESULTS
EcN increases transepithelial resistance (TER) in different intestinal epithelial models
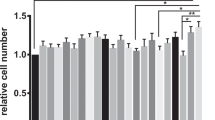
Treatment of HT-29/B6 monolayers with vital E. coli strains for 24 h showed different effects on TER (Figure 1a). Common laboratory strains, such as DH5α, ATCC11229, or VSC257, had no effect on TER or even reduced it slightly. Enteropathogenic E. coli, a typical causative agent of diarrhea, decreased TER. In contrast, probiotic EcN induced an increase in TER. Interestingly, Lactobacillus casei Shirota, another probiotic strain, had no effect on TER (Figure 1a). Comparable TER effects of EcN were also observed in Caco-2 monolayers (Figure 1b). Moreover, two-path impedance measurements performed on colonic specimens obtained from piglets, which underwent supplement feeding with EcN, revealed an increase in paracellular resistance compared with untreated controls (Figure 1c).
Escherichia coli Nissle 1917 (EcN) increases transepithelial resistance (TER) in different models. The impact of EcN on TER was tested in (a) HT-29/B6 monolayers in comparison with other E. coli strains (n≥3) or L. casei Shirota (n=3) and in (b) Caco-2 monolayers (n=4). Changes in TER from initial value are given at 24 h of incubation. (c) Two-path impedance spectroscopy shows that feeding of piglets with EcN supplement promotes increased colonic paracellular resistance (EcN, n=5; control, n=8). Repi, transepithelial electrical resistance; Rpara, paracellular resistance; Rtrans, transcellular resistance. *P<0.05, **P<0.01, ***P<0.001 vs. control.
As it is known that EcN secrets active factors into the culture medium,4, 5 the impact of EcN culture supernatant (EcNsup) on TER was examined. Treatment with EcNsup also increased TER over time (Figure 2a), whereas L. casei Shirota culture supernatant was ineffective (data not shown). The effect of EcNsup on TER could be abolished by intensive heat treatment (EcNsup 95°C, Figure 2a) and was also diminished by proteinase K digestion, suggesting a proteinaceous component (Figure 2a). Subsequently, supernatants of two EcN mutant strains were studied regarding their potential to stimulate TER. Although the supernatant of the ΔfliA mutant strain (ΔfliAsup), which is incapable of flagella gene transcription, increased TER comparable to the wild-type strain, supernatant of the tcpC-deficient strain (ΔtcpCsup) had lost this ability and TER remained at control level within 24 h of incubation (Figure 2b). To assess the beneficial TcpC effect, tcpC from EcN was cloned into the tcpC-negative laboratory E. coli strain TOP10F. TOP10F carrying tcpC from EcN (clone 5) increased TER in HT-29/B6 monolayers compared with wild-type TOP10F treated or untreated control monolayers (Figure 2c).
Influence of Escherichia coli Nissle 1917 (EcN) TcpC on transepithelial resistance (TER). (a) Incubation with EcN culture supernatant (EcNsup) resulted in a time-dependent increase in TER (n=9, *P<0.05, ***P<0.001 vs. control), which was abolished by proteinase K (n=3) or heat treatment (EcNsup95 °C, n=6) of EcNsup. (b) Impact of supernatants prepared from the two EcN mutant strains (ΔfliA and ΔtcpC) on TER compared with effects of EcNsup from wild-type bacteria (n=5; ***P<0.001 vs. EcNsup). (c) E. coli TOP10F carrying E. coli Nissle tcpC gene (clone 5) induces a TER increase in HT-29/B6 monolayers (n=5; **P<0.01 vs. control or TOP10F).
Effect of EcN on intracellular signaling pathways
EcN was recently described to affect the atypical PKC-ζ (PKCζ).23 We found that EcNsup increased PKCζ phosphorylation within 10–20 min and that phosphorylation of extracellular signal-regulated kinases 1/2 (ERK1/2) was induced at 30 min (Figure 3a). Interestingly, the ΔtcpC mutant strain did not stimulate PKCζ or ERK1/2 phosphorylation (Figure 3b, quantification is given in Supplementary Figure S1 online). Involvement of other mitogen-activated protein kinases (p38 or c-Jun N-terminal kinase) or phosphatidylinositol-3-kinase, which are often functionally linked to PKC signaling, was not observed (data not shown).
TcpC alters protein kinase C-ζ (PKCζ) and extracellular-signal-regulated kinase 1/2 (ERK1/2) signaling. (a) Incubation of HT-29/B6 cells with Escherichia coli Nissle 1917 culture supernatant (EcNsup) enhanced phosphorylation of atypical PKCζ (p-PKCζ) within 10 to 20 min and induced ERK1/2 phosphorylation at 30 min after inoculation. (b) Phosphorylation of PKCζ (at 20 min) or (c) ERK1/2 (at 30 min) was not stimulated by ΔtcpCsup. One representative western blot of at least four independent experiments is shown.
Improvement of paracellular barrier function
The following analyses were performed on HT-29/B6 monolayers after 24 h of incubation with EcNsup. If not stated otherwise, untreated monolayers served as control. To study electrophysiological properties, monolayers were mounted into Ussing chambers, and TER and short circuit currents (ISC) were determined and unidirectional flux measurements were performed from mucosal to serosal with [3H]-mannitol (182 Da, radius 3.6 Å). As shown in Table 1, ISC was not affected by EcNsup, which points against a change in a rheogenic transport process contributing to the resistance effect. In support of this hypothesis, the increase in TER was accompanied by a reduction in mannitol permeability.
Two-path impedance spectroscopy was performed to differentiate between trans- and paracellular resistance. EcNsup improved the barrier function predominantly by sealing the paracellular route (Figure 4a). In EcNsup-treated monolayers, paracellular resistance (Rpara) was 3.4-fold higher than in controls and this was the main contribution to the increase in transepithelial electrical resistance (Repi). In contrast, transcellular resistance (Rtrans) contributed only marginally.
Impact on tight junction (TJ) proteins. (a) Two-path impedance spectroscopy revealed that Escherichia coli Nissle 1917culture supernatant (EcNsup) increased paracellular resistance (Rpara), whereas transcellular resistance (Rtrans) was only marginally affected (n=4; ***P<0.001 vs. control). Repi, transepithelial electrical resistance. (b) Patterns of TJ protein expression in untreated controls and EcNsup-treated monolayers. Representative western blots of at least three independent experiments are shown. ZO-1, zonula occludens protein-1. (c) Densitometric quantification was performed and all values were normalized to β-actin (n=6; **P<0.01). (d) Claudin-14 mRNA levels were examined in HT-29/B6 monolayers at 3, 6, 12, and 24 h after EcNsup inoculation. At each time point, mRNA levels are given as means±s.e.m. of x-fold increase over controls (n=8; *P<0.05).
Because this paracellular effect pointed towards TJ changes, expression of several barrier-relevant TJ proteins was studied. In western blot analyses performed after 24 h of EcNsup incubation, upregulation of claudin-14 was noted, whereas protein expression of other TJ proteins was not affected (Figure 4b). Densitometric quantification and normalization to β-actin confirmed an increase in claudin-14 to 169±10% compared with control (Figure 4c). Heat-inactivated EcNsup95 °C did not alter claudin-14 protein level, and the expression level remained similar to that of control monolayers (Supplementary Figure S2 online).
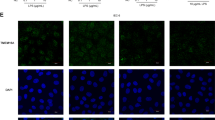
Furthermore, real time quantitative polymerase chain reaction experiments demonstrated transcriptional regulation of claudin-14. As early as 3 h after inoculation with EcNsup, upregulated claudin-14 mRNA expression by ∼3.7±0.8-fold was observed, which then returned to control level. The time course of claudin-14 mRNA expression and parallel TER measurements are given in Figure 4d. Immunofluorescence staining and subsequent confocal laser scanning microscopy revealed enhanced claudin-14 and occludin signals after EcNsup stimulation compared with ΔtcpCsup-treated or control monolayers (Figure 5a). Quantification of claudin-14 TJ signal intensity indicated an increase in claudin-14 to 151±7% compared with control (100%) or 116±23% after ΔtcpCsup treatment. Intracellular claudin-14 signal intensity and cell size were unchanged.
Claudin-14 regulation. (a) Analyses of immunostained claudin-14 (red) and occludin (green) in control, Escherichia coli Nissle 1917 culture supernatant (EcNsup) and ΔtcpCsup-treated monolayers by confocal laser scanning microscopy (LSM). One representative LSM graph (z-stack) is depicted from n=4 independent experiments. (b) Presence of both inhibitors, protein kinase C-ζ-pseudosubstrate (PKCζ-PS) and U0126 blocked EcNsup-mediated transepithelial resistance (TER) increase (n=6; ***P<0.001 vs. EcNsup without inhibitors) and (c) upregulation of claudin-14. One representative western blot of five independent experiments is shown (n=5; *P<0.05, **P<0.01, ***P<0.001 vs. EcNsup without inhibitors). NS, not significant.
However, noticeable changes in the junctional localization of other functionally relevant claudins were not observed (Supplementary Figure S3 online). Finally, the regulatory influence of PKCζ and ERK1/2 signaling on claudin-14 protein expression was analyzed. Therefore, ERK1/2 phosphorylation was inhibited by U0126 and PKCζ activity was blocked by application of a myristoylated pseudosubstrate (PKCζ-PS). Simultaneous inhibition of ERK1/2 and PKCζ activity prevented the EcNsup-induced TER increase (Figure 5b) and claudin-14 upregulation (Figure 5c), whereas single inhibition of these signaling pathways did not (data not shown).
Functional relevance of claudin-14
So far, little is known about the functional role of claudin-14. A recent study investigated claudin-14 function in a kidney cell culture model. Overexpression resulted in a selective decrease in permeability to cations,24 thus proposing a barrier-forming property for claudin-14. Selectivity to ions was also investigated in our present model. In parallel to TER (Figure 6a), Na+ and Cl– dilution potentials were measured in HT-29/B6 after EcNsup incubation. EcNsup reduced permeability to both Na+ and Cl– when compared with controls (Figure 6b). However, the ratio of these permeabilities remained unchanged in HT-29/B6 after EcNsup exposure (Figure 6c). Consequently, EcNsup did not promote selective discrimination of ions but sealed the barrier in HT-29/B6 monolayers similarly against sodium and chloride. To elucidate the putative barrier-forming role of claudin-14 in HT-29/B6 monolayers, expression of claudin-14 was reduced by transient transfection of HT-29/B6 cells with a claudin-14-specific small interfering RNA (cldn-14 siRNA). The time course is given in Figure 7a and shows that TER in claudin-14 siRNA-transfected cell layers amounted to only 53±3% of that in monolayers transfected with green fluorescent protein (GFP) siRNA as control. In parallel, protein expression of claudin-14 was reduced in claudin-14 siRNA-transfected cells compared with monolayers transfected with GFP siRNA (69±9% in claudin-14 siRNA compared with control, Figure 7b).
Influence of Escherichia coli Nissle 1917 culture supernatant (EcNsup) on permeability to Na+ and Cl–. HT-29/B6 monolayers, pretreated with EcNsup for 24 h, were mounted into Ussing chambers. (a) Transepithelial resistance (TER) was measured (n=6–9, **P<0.01) and (b) permeabilities to sodium (PNa) and chloride (PCl) were determined by measurement of dilution potentials (n=6 or 9; PNa: ***P<0.001; PCl: ###P<0.001 vs. control). (c) The permeability ratios PNa/PCl in EcNsup-treated monolayers was not different from that of nonstimulated controls.
Barrier-forming function of claudin-14 (Cldn-14). HT-29/B6 cells were transfected with claudin-14 small interfering RNA (siRNA) or green fluorescent protein (GFP) siRNA as control. (a) The time course of transepithelial resistance (TER) measured at 48, 72, and 96 h after nucleofection of claudin-14 or GFP siRNA indicates a larger increase with GFP siRNA within the first 72 h (n=8 or 10; *P<0.05). (b) TER and claudin-14 expression levels (n=4) obtained at 72 h after nucleofection. Control values were set to 100% (*P<0.05, ***P<0.001). (c) Corresponding western blot of four comparable experiments performed at 72 h after nucleofection.
DISCUSSION
Deterioration of the intestinal barrier has two major consequences: (i) electrolytes and water can leak into the intestinal lumen contributing to diarrhea (leak flux diarrhea) and (ii) increased transmucosal permeation of noxious and/or immunogenic agents may occur.
The “sealing” action of EcN likely improves intestinal barrier function and thus may—at least in part—explain the observed benefits in patients. So far, EcN-induced barrier improvement was attributed to upregulation of ZO proteins.6, 8, 23 However, barrier function is directly dependent on the presence and composition of transmembranal TJ proteins such as the claudins, whereas ZO proteins rather provide scaffold and regulate their interplay.25 Consequently, this study was conducted to examine EcN influence on strand-forming TJ proteins and to elucidate the TJ-modulating bacterial factor.
In contrast to laboratory E. coli strains, enteropathogenic E. coli, or the probiotic strain L. casei Shirota, treatment of HT-29/B6 monolayers with vital EcN or EcNsup resulted in strongly increased TER. This effect was not unique to HT-29/B6 cells as it was confirmed in Caco-2 monolayers. Moreover, we observed effects on Rpara in piglet colon specimens supporting the barrier-stabilizing properties of EcN that were already assumed from clinical studies.1, 2 TER effects on unchallenged epithelial cells were already reported for other vital probiotic preparations, for example, Streptococcus thermophilus or Lactobacillus acidophilus.26 Soluble as well as structurally bound signaling molecules are typical for communication/interaction of the gut microbiota with the host. With respect to EcN, different structures such as flagellin or the capsule polysaccharide are well known to exhibit immune stimulatory properties.5, 27, 28 However, flagellin was not responsible for the EcN-mediated effect demonstrated in this study, because a flagella-defective strain was still effective. However, the TER effect turned out to be mediated by the TcpC protein, as ΔtcpCsup did not stimulate TER anymore. As a further piece of evidence, cloning of EcN tcpC into E. coli TOP10F caused this strain to develop a TER increase in HT-29/B6 monolayers too. These findings are in line with data from a mouse dextran sulfate sodium colitis model where EcN TcpC proved to be an important protective bacterial factor (Julia Frick, Tübingen, personal communication). Originally, TcpC, which refers to TIR domain containing-protein, was characterized in an uropathogenic E. coli strain CFT073. Here, TcpC was secreted and reported to impede TLR signaling through direct binding to the adaptor protein MyD88 with its TIR domain.29 From this work, TcpC was established as a virulence factor for urinary tract infection. So far, the role of TcpC in EcN is not known. As TLR agonists do not lead to an increase in TER in our model, it may well be that the protective effect on epithelial cells seen in our model is mediated by a different mechanism. In this study we found that TcpC regulates the phosphorylation of PKCζ and ERK1/2 in an epithelial cell line. Using T84 cells, Zyrek et al.23 already reported an influence of EcN on PKCζ. In this study, EcN enhanced the expression and translocation of PKCζ from the cytosol to the membrane, but reduced its activity. PKCζ belongs to the atypical protein kinases and acts as a serine/threonine protein kinase. It forms a complex with the cell polarity proteins PAR-6 and PAR-3 that control TJ formation as, for example, shown in MDCK (Madin Darby canine kidney) cells.30 Recently, induction of PAR-3 and PAR-6 mRNA by EcN was reported in T84 cells.7 However, the functional relevance and the bacterial factor of this finding were not clear yet. Activation of PKCζ involves the release of a pseudosubstrate and a phosphorylation in the kinase domain and is influenced by phosphatidylinositol-3,4,5-trisphosphate, which is produced from phosphatidylinositol-4,5-bisphosphate by phosphatidylinositol-3-kinase. In our study, we did not observe any involvement of phosphatidylinositol-3-kinase (data not shown), but EcNsup incubation enhanced the phosphorylation of ERK1/2.
Subsequent analyses of the EcN-induced barrier effect pointed to a TJ contribution. EcNsup reduced mannitol permeability that, because of its size, can pass the paracellular barrier.31 In addition, two-path impedance analysis revealed that the EcN-mediated TER increase is mainly because of increased paracellular resistance. For identifying the nature of this paracellular effect, expression and localization of most of the functionally important TJ strand proteins was studied by western blotting and confocal laser scanning microscopy. Interestingly, in western blots only claudin-14 was affected by EcNsup. Claudin-14 was enhanced at both protein and mRNA levels, pointing to an expression regulation from the gene. This interpretation is also supported by the time sequence of the mRNA. Heat-inactivated EcNsup95 °C did not increase TER or claudin-14 expression. When immunofluorescence staining followed by confocal laser scanning microscopy was performed, enhanced claudin-14 staining was found in the TJs of EcNsup-treated monolayers, compared with control and ΔtcpCsup-incubated monolayers. From this and in line with other studies,7, 23 EcN could also enhance the assembly of TJs by preferentially stimulating the insertion of claudin-14 into TJ strands.
Although western blot did not show any changes in occludin protein expression, immunofluorescence staining appeared to indicate enhanced signals in TJs. The functional relevance of this observation is not clear. From knockout mice it is known that occludin is not obligate for intact barrier function and it was speculated to be dispensable when substituted by other TJ proteins.32 In contrast to other studies performed on diverse cell and animal models,6, 8, 23 we did not see EcNsup to stimulate ZO-1 expression in HT-29/B6. This may simply depend on the model, as it is not unlikely that EcN acts differently under pathological or germ-free conditions or redundancy of ZO proteins is different in our cell model. Enhanced expression of ZO proteins may stabilize the TJs and improve the morphology of damaged epithelia. However, in contrast to strand-forming TJ proteins, ZO scaffold proteins have no direct influence on TER.
Interestingly, we found EcN-induced claudin-14 upregulation to involve PKCζ and ERK1/2 signaling. As neither inhibition of PKCζ nor of ERK1/2 alone abolished the EcNsup effect, simultaneous PKCζ and ERK1/2 inhibition was tested. TER was slightly decreased by the inhibitor combination, indicating that these pathways are relevant for barrier function. Under these conditions, EcNsup was no longer able to induce a significant TER increase. In fact, regulation of claudin-14 depended on the interplay of both signaling components, e.g. because of parallel signaling pathways toward claudin-14 within the TJ. PKCζ is involved in the mitogen-activated protein kinase cascade in a variety of cells.33, 34, 35 In a recent study the influence of ERK1/2 on TJ integrity was dependent on cell differentiation status. Although ERK1/2 destabilizes the TJs in immature Caco-2 cell layers, it mediates protection and stabilization of the TJs in fully differentiated cells. In the latter case, epidermal growth factor stimulated PKCζ and enhanced its localization at the TJs, which was ERK dependent.36 Moreover, the ability to stimulate PKC/ERK1/2 signaling seems not to be restricted to EcN. For example, secretory proteins of probiotic Lactobacillus rhamnosus GG were observed to protect intestinal epithelial TJs from hydrogen peroxide damage in a PKC- and mitogen-activated protein kinase-dependent manner.37
Although deletion of claudin-14 is known to be associated with deafness,38, 39 its function in the intestine is unknown. Expression of claudin-14 in a leaky canine kidney cell line (MDCK-II) resulted in a TER increase, pointing to sealing properties.24 This is in accordance with the results obtained from our siRNA transfection approach in this study. Here diminished claudin-14 protein expression was accompanied by TER reduction. However, selective discrimination against sodium as described for claudin-14 overexpression in MDCK-II24 could not be observed in HT-29/B6 cells. EcNsup reduced sodium and chloride permeabilities to a similar extent. Probably, also this experimental difference is cell model specific and may reflect the different specific background of TJ proteins of both cell lines. For example, in contrast to HT-29/B6, MDCK-II cells possess a high expression level of claudin-2 that acts as selective cation channel.40
EcN exerts a multimodal effect on the epithelial gut barrier. Beside other mechanisms, its probiotic properties comprise an epithelial barrier strengthening function. From this study we obtained experimental evidence that supernatant from E. coli Nissle lacking tcpC (i) has lost its ability to enhance TER, (ii) was unable to stimulate phosphorylation of PKCζ and ERK1/2, and (iii) accordingly did not induce claudin-14 upregulation. Thus, we conclude that the increase in TER on HT-29/B6 monolayers is mediated by the TcpC protein of EcN through PKCζ and ERK1/2 signaling. Claudin-14 has barrier-forming properties and contributes to EcN-induced barrier improvement.
METHODS
Cell culture
The colon carcinoma cell line HT-29/B6, a subclone of the human colon carcinoma cell line HT-29, is an appropriate model to study TER, reflecting barrier integrity.41 Culture medium contained RPMI-1640 (PAA Laboratories GmbH, Pasching, Austria) for HT-29/B6 or MEM+GlutaMax (Life Technologies GmbH, Darmstadt, Germany) for Caco-2, 15% fetal calf serum, and 1% penicillin/streptomycin. Cells were incubated at 37 °C in an air atmosphere containing 5% CO2. For experimental analyses, cells were seeded on Millicell PCF filter supports (effective area: 0.6 cm2; 3 μm pores Millicell PCF, Millipore, Schwalbach, Germany) and experiments were performed when confluent monolayers were fully polarized.
Construction of EcN ΔTcpC gene knockout
To selectively remove the TcpC gene from the chromosome of EcN strain, the single step gene knockout procedure was performed, as described by Datsenko and Wanner.42 In brief, oligonucleotide primers were designed for introducing a kanamycin resistance gene cassette, so that the open reading frame of the TcpC gene was selectively replaced by a kanamycin resistance gene. Primers used were: (i) P1.Tir.for: 5′-TAGTAATGTTATCCCCACTATTAATTTTTAATCAAATGCATGTTACATAGGCTCAGTGTAGGCTGGAGCTGCTTC-3′ and (ii) P2.Tir.rev: 5′-CACGGAACACTCATTCCATAACAAACATACTATTTAGATGTGCTAATACAATTAATCATATGAATATCCTCCTTA-3′. Annealing regions of 20 bp homologous to the kanamycin resistance cassette template plasmid, pKD4, were included as well as 55 bp of sequence homologous to regions flanking the EcN TcpC gene. The purified PCR product obtained was subsequently electroporated into EcN, which had previously been transformed with the lambda Red recombinase expression vector, pKD46 bearing a heat-labile origin. To facilitate recombination of the electroporated fragment, expression of lambda Red recombinase in EcN cells was induced by treatment with 100 mM arabinose at 27 °C. After the transformation of the PCR fragment into EcN (pKD46), cells were recovered in Luria Broth at 37 °C under agitation. Kanamycin-resistant transformants were grown at 37 °C to induce the loss of the heat-labile pKD46 plasmid. Loss of pKD46 plasmid was confirmed by loss of resistance against Ampicillin. Final knockout isolates were screened by PCR for the absence of the TcpC gene and the presence of the kanamycin cassette. Further confirmation was performed by sequencing of the upstream and downstream insertion sites.
Construction of E. coli TOP10F EcN TcpC strain
To artificially introduce a functional copy of TcpC derived from the original strain EcN into K-12 strain Top10F, the respective region from the chromosome of EcN was amplified using the oligonucleotide primers SerU-Island.10670.for 5′-GCAACGCATATGATAGTAAT-3′ and tcpC.LongRekomp.rev 5′-CAATATGTGGAATGAGCA-3′. The amplified 3.5-kb fragment was cloned into the PCR4 TOPO vector by means of TA cloning (Life Technologies GmbH) according to the manufacturer’s instructions. Confirmatory sequencing was performed. The plasmid was subsequently isolated and transformed into TOP10F electrocompetent cells.
EcN piglet feeding trial
Piglets (28 days of age) were fed a standard chow and water ad libitum supplemented either with or without EcN every second day (1 × 109 CFU given by oral gavage, controls received saline). After 2 weeks, the colon was removed and specimens were partially stripped and inserted into Ussing-type chambers. One- and two-path impedance spectroscopy were performed as described below.
Preparation of EcN supernatant
EcN was grown in 4 ml Luria-Bertani Broth (MP Biomedicals, Illkirch, France) overnight at 37 °C. The next day, 20 ml Luria-Bertani Broth was inoculated from overnight culture to an optical density (OD5578 nm) of 0.4. Bacteria were cultured to an OD578 of at least 1 and were used for preparation of a further culture, starting with an OD578 of 0.3. When an OD578 above 1 was reached, Luria-Bertani Broth was removed by centrifugation at 5,000 × g and the pellet was washed with RPMI-1640 medium without phenol red (PAA Laboratories GmbH). The bacterial pellet was resuspended to an OD578 of 2. From this a preparative culture was inoculated to an OD578 of 0.3 and EcN was grown in RPMI to an OD578 of 1.5. This preparative culture was centrifugated at 8,000 × g for 15 min at 4 °C. The supernatant was filtrated using Sterivex-GV 0.22 μm (Millipore) and afterwards concentrated using Centricon Plus-20 centrifugal filters (Millipore) with a molecular weight cutoff MWCO of 100 kDa.
Treatment of HT-29/B6 monolayers with bacteria or supernatant
For infection procedures with viable bacteria, monolayers were shifted to serum and antibiotic-free RPMI medium 2 h before experiments. All E. coli strains were grown in Luria Bertani Broth, and EcN mutant strains were cultured in the presence of 50 μg ml−1 kanamycin. L. casei Shirota was raised under anaerobic conditions in de Man, Rogosa, and Sharpe medium (Roth, Karlsruhe, Germany).
A 1:8 dilution of the bacterial overnight culture was incubated for additional 1.5 h at 37 °C. Bacteria were harvested from log-phase cultures, washed, and resuspended in RPMI or MEM+GlutaMax. Cells were treated with 107 or 108 vital bacteria from the apical side equaling a multiplicity of infection of 10 or 100. Incubation was carried out at 37 °C in an atmosphere of 95% O2 and 5% CO2 for 24 h. To avoid overgrowth, bacteria were killed by the addition of 100 μg ml−1 gentamicin at 3 h after inoculation. All investigated strains are listed in Table 2.
Before treatment with supernatant, HT-29/B6 cells were placed into serum-free RPMI medium containing 100 μg ml−1 gentamicin. Then, 10 μl of EcNsup, ΔtcpCsup, or ΔfliAsup were added to the apical compartment. In parallel, two types of controls were performed: (i) without adding EcN or EcNsup and (ii) addition of heat-treated EcNsup (carried out at 95 °C for 15 min). Proteinase K digestion of EcNsup was carried out with 2 μg ml−1 of proteinase K (TUNEL, In Situ Cell Death Detection Kit, Fluorescein, Roche, Mannheim, Germany) for 2 h at 37 °C.
Signaling pathways were examined by preincubation of HT-29/B6 monolayers with 1 mM protein kinase C-zeta pseudosubstrate, myristoylated (Life Technologies GmbH) and 10 μM U0126 (Calbiochem, Darmstadt, Germany). EcNsup was added after 4 h of pretreatment.
Measurement of TER
TER (Ω cm2) was determined with an ohmmeter (D. Sorgenfrei, Institute of Clinical Physiology, Berlin, Germany) under sterile conditions at 37 °C. Resistance values were corrected for the resistance of the bathing fluid between the voltage-sensing electrodes and the empty filter.
Flux measurement
At 24 h after infection, HT-29/B6 cell monolayers were mounted into Ussing chambers. TER was determined by a computerized automatic clamp device (Fiebig Hard & Software, Berlin, Germany) and unidirectional flux measurement was performed from mucosal to serosal under short circuit conditions with [3H]-mannitol (Biotrend, Cologne, Germany) according to previously described protocols.45 The bathing solution contained nonlabeled mannitol (10 mM). Then, [3H]-mannitol was added to the mucosal side and samples were taken from the basolateral chamber at 0, 15, 30, 45, and 60 min. Ultima Gold high flash-point liquid scintillation cocktail (PerkinElmer LAS GmbH, Rodgau-Juegesheim, Germany) was used for analysis in a Tri-Carb 2100TR liquid scintillation counter (Packard, Frankfurt, Germany).
Two-path impedance spectroscopy
Repi, Rtrans, and Rpara of HT-29/B6 monolayers were measured by two-path impedance spectroscopy as recently published.45 Briefly, filters were mounted in Ussing-type impedance chambers and bathed with Ringer’s solution. Sinusoidal alternate currents at frequencies from 1 to 65 kHz were provided by an electrochemical interface (1286, Solartron Schlumberger, Farnborough, UK), connected to a phase-sensitive multimeter (PSM 1700 PsimetriQ, Newtons4th, Loughborough, UK). In addition, fluxes of the paracellular marker fluorescein were measured. Change of Rpara was induced by Ca2+ removal using EGTA and the resulting impedance spectra and fluxes before and after chelating extracellular Ca2+ were used for calculation.
Confocal laser scanning microscopy
After an incubation period of 24 h, HT-29/B6 monolayers were washed and fixed with methanol for 1 h at −20 °C followed by an aceton treatment of 5 min. Afterwards, monolayers were rehydrated with immunofluorescence medium (0.1% Triton X-100, 0.15 M NaCl, 5 mM EDTA, 20 mM HEPES, pH 7.5, 0.02% NaN3) for 10 min at room temperature. TJ proteins were immunostained with antibodies against ZO-1 (1:500; Zymed, San Francisco, CA), occludin, claudin-3, -4, -5, -8, (1:200; Zymed) and claudin-14 (1:500; LifeSpan BioSciences, Seattle, WA), followed by incubation with Alexa Fluor 488 goat anti-rabbit IgG, Alexa Fluor 594 goat anti-mouse and/or Alexa Fluor 488 rabbit anti-goat IgG (1:500, Life Technologies GmbH). Immunostaining of the TJ meshwork was visualized by confocal laser scanning microscopy (Zeiss LSM 510, Jena, Germany). For quantification of signal intensity, projections of z-stacks were generated and LSM510 Version 3.2 SP2 software tools were used to calculate TJ signal intensity and to measure cell size.
Western blot
Protein expression was analyzed using the western blot technique as described previously.46 At 24 h after treatment, whole-cell extracts were generated with ice-cold lysis buffer including 10 mM Tris, pH 7.5, 150 mM NaCl, 0.5% Triton X-100, and 0.1% SDS. For detection of ZO proteins, cells were lysed by ultrasonification using a buffer including 25 mM Hepes, 20 mM EDTA, 25 mM NaF, 1% SDS, and complete protease inhibitor mixture (Roche). To check for phosphorylation, lysis buffer contained 20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM Na2H2P2O7, 1 mM β-glycerolphosphate, 1 mM Na3VO4, 1 μg ml−1 leupeptin, and 1 mM PMSF. Before use, complete protease inhibitor mixture (Roche) was added.
Protein concentrations were determined by Pierce BCA assay (Thermo Scientific, Ulm, Germany) and 15–25 μg of whole cell extracts were loaded on polyacrylamid gels. For immunodetection the following antibodies were used: anti-occludin and anti ZO-1 (1:2,000, Zymed), anti-tricellulin (customized by the group of O. Huber, Institute of Clinical Chemistry and Pathobiochemistry, Charité, Germany.), anti-claudin-1 -5, -7, -8, and -10 (1:1,000, Zymed), anti-claudin-14 (1:1,000, LifeSpan BioSciences, SEA), anti-p-PKCζ(Thr410)-R (1:200, Santa Cruz Biotechnology, Heidelberg, Germany), anti-PKCζ (1:1,000, Cell Signaling Technology, Frankfurt am Main, Germany), p-p44/42 and p44/42 (1:1,000, Cell Signaling Technology), and anti-β-actin (1:5,000, Sigma-Aldrich, Schnelldorf, Germany) as internal loading control.
Measurement of Na+ and Cl– permeability
At 24 h after incubation, EcNsup-treated monolayers and controls were mounted into Ussing chambers and water-jacketed gas lifts were filled with 10 ml circulating Ringer’s solution (in mM: Na+ 140; Cl– 149.8; K+ 5.4; Ca2+ 1.2; Mg2+; HEPES 10 and D(+)-glucose; pH 7.4). The TER effect on pretreated monolayers was stabilized by the addition of 30 μl of EcNsup to the apical compartment. Sab simplex (Pfizer Pharma GmbH, Berlin, Germany) was used to reduce surface tension. To avoid contribution through altered ion channel activity, NaCl dilution potentials were measured at 14 °C. Briefly, voltage and TER (Ω cm2) were monitored and one hemichamber was switched to a solution containing a reduced concentration of NaCl, whereas all other components were identical to standard Ringer’s solution. Osmolarity was balanced by mannitol. NaCl permeability was determined from dilution potentials and the Goldmann–Hodgkin–Katz equation as reported previously.40, 47, 48
siRNA transfection assay
For transfection of HT-29/B6 cells with claudin-14 siRNA, the Amaxa biosystems siRNA kit (Lonza Cologne AG, Cologne, Germany) was used. A total of 1 × 106 cells were transfected with 10 μl of claudin-14 siRNA (Santa Cruz, Heidelberg, Germany) or 5 μl maxGFP siRNA (Lonza Cologne AG) as vector control according to the manufacture’s protocol. After transfection, cells were immediately seeded on Millicell PCF filters and the course of TER was monitored for ∼3 days.
Real time quantitative polymerase chain reaction
Total RNA was extracted from HT-29/B6 cells by RNAzol (Invitrogen) following the manufacturer’s protocol. For reverse-transcription PCR, High-Capacity cDNA Archive Kit (Applied Biosystems, Mannheim, Germany) was used. Real-time PCR was carried out using TaqMan Gene Expression Assay no Hs00273267_s1 (Applied Biosystems) for human claudin-14. Human glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Applied Biosystems) served as endogenous control. Claudin-14 and GAPDH complementary DNAs were quantified via VIC and FAM reporter dyes covalently attached to the 5'-terminal base of the probes. PCR was set up in duplicates, and threshold cycle (Ct) values of the target genes were normalized to the endogenous control. Differential expression was calculated according to the 2−ΔΔCT method.49
Statistical analyses
All data are expressed as means±s.e.m. Statistical analysis was performed using Student’s t-test and one-way analysis of variance testing in case of multiple testing adjusted by Bonferroni–Holm correction. P<0.05 was considered significant (*P<0.05, **P<0.01, ***P<0.001).
References
Rembacken, B.J., Snelling, A.M., Hawkey, P.M., Chalmers, D.M. & Axon, A.T. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet 354, 635–639 (1999).
Kruis, W. et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 53, 1617–1623 (2004).
Altenhoefer, A. et al. The probiotic Escherichia coli strain Nissle 1917 interferes with invasion of human intestinal epithelial cells by different enteroinvasive bacterial pathogens. FEMS Immunol. Med. Microbiol. 40, 223–229 (2004).
Wehkamp, J. et al. NF-kappaB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic bacterium. Infect. Immun. 72, 5750–5758 (2004).
Schlee, M. et al. Induction of human beta-defensin 2 by the probiotic Escherichia coli Nissle 1917 is mediated through flagellin. Infect. Immun. 75, 2399–2407 (2007).
Cichon, C., Enders, E., Sonnenborn, U. & Schmidt, M.A. DNA-microarray-based comparison of cellular responses in polarized T84 epithelial cells triggered by probiotics: E. coli Nissle and Lactobacillus acidophilus PZ1041. Gastroenterology 126, A579 (2004).
Veltman, K., Hummel, S., Cichon, C., Sonnenborn, U. & Schmidt, M.A. Identification of specific miRNAs targeting proteins of the apical junctional complex that simulate the probiotic effect of E. coli Nissle 1917 on T84 epithelial cells. Int. J. Biochem. Cell Biol. (2011).
Ukena, S.N. et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One 2, e1308 (2007).
Anderson, J.M. Molecular structure of tight junctions and their role in epithelial transport. News Physiol. Sci. 16, 126–130 (2001).
Tsukita, S., Furuse, M. & Itoh, M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2, 285–293 (2001).
Turner, J.R. ‘Putting the squeeze’ on the tight junction: understanding cytoskeletal regulation. Semin. Cell Dev. Biol. 11, 301–308 (2000).
Gonzalez-Mariscal, L., Betanzos, A. & Avila-Flores, A. MAGUK proteins: structure and role in the tight junction. Semin. Cell Dev. Biol. 11, 315–324 (2000).
Mitic, L.L., Van Itallie, C.M. & Anderson, J.M. Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: lessons from mutant animals and proteins. Am. J. Physiol. Gastrointest. Liver Physiol. 279, G250–G254 (2000).
Furuse, M. et al. Occludin: a novel integral membrane protein localizing at tight junctions. J. Cell Biol. 123, 1777–1788 (1993).
Furuse, M., Fujita, K., Hiiragi, T., Fujimoto, K. & Tsukita, S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 141, 1539–1550 (1998).
Ikenouchi, J., Furuse, M., Furuse, K., Sasaki, H. & Tsukita, S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J. Cell Biol. 171, 939–945 (2005).
Gonzalez-Mariscal, L., Tapia, R. & Chamorro, D. Crosstalk of tight junction components with signaling pathways. Biochim. Biophys. Acta. 1778, 729–756 (2008).
Fujibe, M. et al. Thr203 of claudin-1, a putative phosphorylation site for MAP kinase, is required to promote the barrier function of tight junctions. Exp. Cell Res. 295, 36–47 (2004).
Clarke, H., Marano, C.W., Peralta Soler, A. & Mullin, J.M. Modification of tight junction function by protein kinase C isoforms. Adv. Drug Deliv. Rev. 41, 283–301 (2000).
Mankertz, J. et al. TNFalpha up-regulates claudin-2 expression in epithelial HT-29/B6 cells via phosphatidylinositol-3-kinase signaling. Cell Tissue Res. 336, 67–77 (2009).
Ewaschuk, J.B. et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G1025–G1034 (2008).
Mennigen, R. et al. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1140–G1149 (2009).
Zyrek, A.A. et al. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCzeta redistribution resulting in tight junction and epithelial barrier repair. Cell. Microbiol. 9, 804–816 (2007).
Ben-Yosef, T. et al. Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration. Hum. Mol. Genet. 12, 2049–2061 (2003).
Fanning, A.S. & Anderson, J.M. Zonula occludens-1 and -2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann. NY Acad. Sci. 1165, 113–120 (2009).
Resta-Lenert, S. & Barrett, K.E. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut 52, 988–997 (2003).
Hafez, M., Hayes, K., Goldrick, M., Grencis, R.K. & Roberts, I.S. The K5 capsule of Escherichia coli strain Nissle 1917 is important in stimulating expression of Toll-like receptor 5, CD14, MyD88, and TRIF together with the induction of interleukin-8 expression via the mitogen-activated protein kinase pathway in epithelial cells. Infect. Immun. 78, 2153–2162 (2010).
Hafez, M. et al. The K5 capsule of Escherichia coli strain Nissle 1917 is important in mediating interactions with intestinal epithelial cells and chemokine induction. Infect. Immun. 77, 2995–3003 (2009).
Cirl, C. et al. Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain-containing proteins. Nat. Med. 14, 399–406 (2008).
Suzuki, A. et al. Atypical protein kinase C is involved in the evolutionarily conserved par protein complex and plays a critical role in establishing epithelia-specific junctional structures. J. Cell Biol. 152, 1183–1196 (2001).
Watson, C.J., Rowland, M. & Warhurst, G. Functional modeling of tight junctions in intestinal cell monolayers using polyethylene glycol oligomers. Am. J. Physiol. Cell Physiol. 281, C388–C397 (2001).
Schulzke, J.D., Gunzel, D., John, L.J. & Fromm, M. Perspectives on tight junction research. Ann. NY Acad. Sci. 1257, 1–19 (2012).
Berra, E. et al. Evidence for a role of MEK and MAPK during signal transduction by protein kinase C zeta. EMBO J. 14, 6157–6163 (1995).
Schonwasser, D.C., Marais, R.M., Marshall, C.J. & Parker, P.J. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol. Cell. Biol. 18, 790–798 (1998).
Monick, M.M., Carter, A.B., Flaherty, D.M., Peterson, M.W. & Hunninghake, G.W. Protein kinase C zeta plays a central role in activation of the p42/44 mitogen-activated protein kinase by endotoxin in alveolar macrophages. J. Immunol. 165, 4632–4639 (2000).
Aggarwal, S., Suzuki, T., Taylor, W.L., Bhargava, A. & Rao, R.K. Contrasting effects of ERK on tight junction integrity in differentiated and under-differentiated Caco-2 cell monolayers. Biochem. J. 433, 51–63 (2011).
Seth, A., Yan, F., Polk, D.B. & Rao, R.K. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G1060–G1069 (2008).
Wilcox, E.R. et al. Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell 104, 165–172 (2001).
Wattenhofer, M. et al. Different mechanisms preclude mutant CLDN14 proteins from forming tight junctions in vitro. Hum. Mutat. 25, 543–549 (2005).
Amasheh, S. et al. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell. Sci. 115, 4969–4976 (2002).
Kreusel, K.M., Fromm, M., Schulzke, J.D. & Hegel, U. Cl- secretion in epithelial monolayers of mucus-forming human colon cells (HT-29/B6). Am. J. Physiol. 261, C574–C582 (1991).
Datsenko, K.A. & Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97, 6640–6645 (2000).
Schierack, P. et al. E. coli Nissle 1917 affects Salmonella adhesion to porcine intestinal epithelial cells. PLoS One 6, e14712 (2011).
Bugarel, M., Martin, A., Fach, P. & Beutin, L. Virulence gene profiling of enterohemorrhagic (EHEC) and enteropathogenic (EPEC) Escherichia coli strains: a basis for molecular risk assessment of typical and atypical EPEC strains. BMC Microbiol. 11, 142 (2011).
Krug, S.M., Fromm, M. & Gunzel, D. Two-path impedance spectroscopy for measuring paracellular and transcellular epithelial resistance. Biophys. J. 97, 2202–2211 (2009).
Amasheh, S. et al. Contribution of claudin-5 to barrier properties in tight junctions of epithelial cells. Cell Tissue Res. 321, 89–96 (2005).
Gunzel, D. et al. Claudin-10 exists in six alternatively spliced isoforms that exhibit distinct localization and function. J. Cell Sci. 122, 1507–1517 (2009).
Yu, A.S. Molecular basis for cation selectivity in claudin-2-based pores. Ann. NY Acad. Sci. 1165, 53–57 (2009).
Livak, K.J. & Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 (2001).
Acknowledgements
This work was supported by DFG Research Unit FOR 721 and SFB 852. We thank Dr Susanne M Krug for analyzing the two-path impedance spectroscopy data and we appreciate the excellent technical assistance of Detlef Sorgenfrei and In-Fah M Lee.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Supplementary information
Rights and permissions
About this article
Cite this article
Hering, N., Richter, J., Fromm, A. et al. TcpC protein from E. coli Nissle improves epithelial barrier function involving PKCζ and ERK1/2 signaling in HT-29/B6 cells. Mucosal Immunol 7, 369–378 (2014). https://doi.org/10.1038/mi.2013.55
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2013.55
This article is cited by
-
Anti-rotavirus Properties and Mechanisms of Selected Gram-Positive and Gram-Negative Probiotics on Polarized Human Colonic (HT-29) Cells
Probiotics and Antimicrobial Proteins (2023)
-
Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier
Microbial Cell Factories (2020)
-
Intestinal effect of the probiotic Escherichia coli strain Nissle 1917 and its OMV
Journal of Diabetes & Metabolic Disorders (2020)
-
Extracellular vesicles and soluble factors secreted by Escherichia coli Nissle 1917 and ECOR63 protect against enteropathogenic E. coli-induced intestinal epithelial barrier dysfunction
BMC Microbiology (2019)
-
Probiotic delivery systems: a brief overview
Journal of Pharmaceutical Investigation (2016)