Abstract
Immunity to Nippostrongylus brasiliensis reinfection requires pulmonary CD4+ T-cell responses. We examined whether secondary lymphoid recruited or pre-existing lung CD4+ T-cell populations coordinated this immunity. To do this, we blocked T-cell egress from lymph nodes using Fingolimod (FTY720). This impaired host ability to resolve a primary infection but did not change effectiveness of recall immunity. Associated with this effective recall immunity was the expansion and T helper type 2 polarization of a pre-existing pulmonary CD4+ T-cell population. LTβR-Ig (lymphotoxin beta-receptor fusion protein)-mediated disruption of stromal cell organization of immune cells did not disrupt this recall immunity, suggesting that protection was mediated by a pulmonary interstitial residing CD4+ T-cell population. Adoptive transfer of N. brasiliensis-experienced pulmonary CD4+ T cells from FTY720-treated wild-type or T-cell interleukin (IL)-4Rα-deficient mice demonstrated protection to be IL-4Rα dependent. These results show that pre-existing CD4+ T cells can drive effective recall immunity to N. brasiliensis infection independently of T-cell recruitment from secondary lymphoid organs.
Similar content being viewed by others
Introduction
Parasitic helminth infections are a major global health problem; a third of all humans are infected at any one time, and infections are a major cause of morbidity. Although drug treatments are effective, they do not provide long-term protection, and infections do not result in robust protective immunity. Detailed understanding of host immunity to helminthes is therefore likely to be critical to future vaccine design.1
T helper type 2 (Th2) immune responses underlie both effective host immunity to helminth infections2, 3 and the onset of allergic inflammatory diseases.4 Development of Th2 immunity typically requires interleukin (IL)-4 and IL-13 signaling via receptors containing an IL-4Rα subunit. This activates effector responses that include goblet cell hyperplasia, Type 2 immunoglobulin (Ig) production, and secretion of the cytokines IL-4, IL-5, IL-9, and IL-13.5 In primary infections, IL-4Rα expression on non-hematopoietic cells is required to drive protection.2, 6, 7 Reinfection studies with Nippostrongylus brasiliensis8 and Heligmosomoides polygyrus9 have demonstrated enhanced Th2 immunity rapidly clearing infection. This protection is strongly related to CD4+ T-cell activation with effector memory T cell and central memory T cell subsets being particularly important.10 Additionally, depletion of CD4+ T cells significantly impaired host antibody responses to reinfection.11 Any specific hematopoietic cellular requirements for IL-4Rα expression following N. brasiliensis reinfection have not been demonstrated.
CD4+ T-cell populations resident in peripheral sites can profoundly influence the coordination of host immune responses.12, 13, 14, 15 Such peripheral CD4+ T-cell populations have important roles in immunity to parasitic nematodes. In Trichuris muris infection, protective immunity is driven by gut-associated lymphoid tissue CD4+ central memory T cells and effector memory T cells.16 Host responses to pulmonary infections can also result in the formation of peripheral lymphoid-like structures/effector lymphoid tissues, which can effectively coordinate host immunity at the site of infection.17
Recall immunity to N. brasiliensis has been associated with lung-initiated CD4+ T-cell responses.8 Whether this is mediated by a pre-existing pulmonary IL-4Rα-expressing CD4+ T-cell population is unknown. In this study, we inhibited lymphocyte movement with Fingolimod (FTY720), an immunomodulator that acts as an agonist to the Sphingosine-1-Phosphate receptor, inducing lymphopenia by blocking lymphocyte egress from secondary lymphoid organs (SLO) to peripheral sites.12, 18, 19 This allowed us to test whether a pre-existing pulmonary CD4+ T-cell population were able to coordinate protective secondary Th2 immunity.
We show that immunity to primary N. brasiliensis infection requires T-cell movement, but Th2 protective responses to secondary infection are independent of T-cell migration. We demonstrate a lung-resident CD4+ T-cell population to be sufficient to confer an IL-4Rα-dependent protection against N. brasiliensis reinfection. Furthermore, we show that the protective lung-resident T-cell population is independent of formation of tertiary lymphoid structures in the lung and is therefore likely to be residing in the lung interstitium.
Results
Migration of lymphocytes is required for protection against primary N. brasiliensis infection
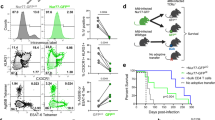
T-cell lymphopenia was induced in mice by daily FTY720 treatment. Lymphopenia was confirmed by tail bleeding and staining whole blood with anti-CD3 and anti-CD4 antibodies. At 24 h post initial FTY720 treatment, circulating CD3+CD4+ T cells decreased from approximately 8.5% to 0.1% of total cells in the blood (Figure 1a). This reduction was maintained following N. brasiliensis infection (Figure 1b).
Treatment with FTY720 induces lymphopenia. (a) Lymphopenia was established by flow cytometric analysis of CD3+CD4+ T cells in whole blood 24 h post FTY720 treatment. (b) Acute lymphopenia was rapidly induced by FTY720 and maintained following Nippostrongylus brasiliensis infection. N=6 mice per group.
To demonstrate requirements for T-cell egress from immune organs in the resolution of primary N. brasiliensis infection, control and FTY720-treated mice were infected with N. brasiliensis. Mice were killed at either 2 or 10 days post infection (PI), and intestinal worm burdens were quantified at day 10 PI (Figure 2a). Control mice had resolved infection, whereas FTY720-treated mice showed high intestinal worm burdens, indicative of a severe impairment in their ability to resolve infection (Figure 2b). It has previously been demonstrated that pulmonary CD4+ T-cell responses closely relate to resolution of primary N. brasiliensis infection.20 To confirm whether impaired protection in FTY720-treated mice related to reduced peripheral T-cell numbers, we quantified T-cell subpopulations in the lung at day 2 and 10 PI. Significantly reduced numbers of CD3+CD4+, CD3+CD8+ T cells (Figure 2cA) and CD4+CD44loCD62Lhi naive, CD4+CD44hiCD62Llo effector memory T cells were found in the FTY720-treated group compared with control (Figure 2cB). A feature of induction of Th2 immunity is upregulation of the IL-1 receptor-related molecule T1/ST2 on effector CD4+ T cells.21 Untreated mice showed significantly increased numbers and a 10-fold increase in the proportions of pulmonary CD4+CD62LloT1/ST2+ T-cell populations (Figure 2d) when compared with the FTY720-treated group. Untreated groups showed increased numbers of CD4+ effector T-cell populations at day 10 when compared with day 2 PI. This was associated with increased T1/ST2 expression and IL-13 cytokine levels.
Migration of T cells to the lung is required for protection against primary Nippostrongylus brasiliensis (NB) infection. (a) Mice were treated daily with FTY720 beginning 1 day before infection and killed either at day 2 or day 10 post infection (PI). (b) Impaired resolution of infection in FTY720-treated mice was established by quantification of intestinal adult worm burdens at day 10 post infection. Flow cytometric analysis of cell suspension of whole lung stained for (cA) CD3+CD4+, CD3+CD8+, (cB) CD4+CD44loCD62Lhi naive, CD4+CD44hiCD62Llo effector memory, and (d) CD4+CD62LloT1/ST2+ T cells. Lung cells were re-stimulated with N. brasiliensis excretory secretory proteins for 5 days, and (e) secretion of interleukin (IL)-13 was detected by enzyme-linked immunosorbent assay. Data are representative of two individual experiments. N=6–10 mice per group. *P<0.05, **P<0.01, ***P<0.001.
Analysis of pulmonary cytokine production in response to N. brasiliensis infection by N. brasiliensis excretory secretory protein–stimulated whole-cell preparations revealed significantly impaired IL-13 secretion in FTY720-treated mice at days 2 and 10 PI (Figure 2e). These findings show a requirement for CD4+ T-cell migration from SLO to be important for host resolution of primary N. brasiliensis infection.
Lung-resident CD4+ T cells are sufficient for protection against secondary N. brasiliensis infection
CD4+ T cells coordinate control of N. brasiliensis reinfection in the lung8 and effective basophil IL-4 production in the lung is required by interaction with CD4+ T cells following primary N. brasiliensis infection.22 We investigated whether T-cell recruitment to lung was required for this protective immunity to secondary N. brasiliensis infection. Mice were treated daily with FTY720 during the period of primary infection only (Supplementary Fig 2 online) or secondary infection only (Supplementary Fig 3 online), as well as throughout primary and secondary N. brasiliensis infection (Figure 3a). This allowed us to dissect whether T-cell recruitment was required at specific time periods for control of secondary infection. At 2 and 5 days post primary and secondary infection, respective lung (Figure 3b) and intestinal worm burdens (Figure 3c) were quantified. Interestingly, equivalent numbers of worms were found in FTY720-treated and untreated mice following secondary infection as opposed to primary infection, where higher worm burdens were observed in the FTY720-treated group. Protection occurred irrespective of the significantly reduced T-cell numbers, including CD4+ effector memory T cells (Figure 3d) and reduced pulmonary IL-4 and IL-13 cytokine production (Figure 3e) in FTY720-treated mice at day 5 post secondary infection. Immuno-histological analysis of CD3+ cell distribution in the lungs of untreated mice following secondary infection showed foci of CD3+ cells at peri-bronchiolar sites and a uniform CD3+ distribution in interstitial tissue. However, in FTY720-treated mice, a significantly reduced localization of CD3+ cells were observed around the peri-bronchiolar and interstitial regions when compared with the untreated group (Figure 3f).
Lung-resident CD4+ T-cell population are sufficient to confer protection against secondary Nippostrongylus brasiliensis (NB) infection. (a) FTY720-treated and untreated mice were given a primary (1o) or secondary (2o) N. brasiliensis infection. Primary infections were cleared at day 7 by Ivermectin treatment, and mice were subsequently reinfected at day 28 and killed at day 2 or day 5 post infection (PI). Ability to resolve infection was established by quantification of (b) lung worm burdens and (c) intestinal worm burdens at day 2 and day 5 post infection, respectively. (d) Flow cytometric analysis of cell suspension of whole lung stained for CD3+CD4+, CD3+CD8+, CD4+CD44loCD62Lhi naive, and CD4+CD44hiCD62Llo effector memory T cells at 5 days post secondary infection. Whole-cell preparations of lung were re-stimulated with N. brasiliensis excretory secretory proteins for 5 days, and (e) cytokine secretion for interleukin (IL)-4 and IL-13 was detected by enzyme-linked immunosorbent assay. (f) Confocal microscopic images of frozen sections of the lung stained with antibody to CD3 (green) and counterstained with Hoechst (blue) around the peri-bronchial and interstitial areas of the lung. White arrows indicate CD3+ T cells. Bar=50 μm (data are representive of one individual experiment, N=8 mice per group). Numbers in the flow cytograms indicate the percentage of T cells present relative to total cell numbers. Data are representative of two individual experiments. N=6–10 mice per group. *P<0.05, **P<0.01.
These results indicate that a small interstitially residing lung CD4+ T-cell population is sufficient to mount effective protective immunity to secondary N. brasiliensis infection.
Lung-resident CD4+ T-cell-driven immunity to N. brasiliensis is independent of tertiary lymphoid structures
If CD4+ T-cell-mediated protection is mediated by interstitially residing T cells, this would suggest that localization of these cells in an organized immune structure such as bronchial-associated lymphoid tissue (BALT) is not required to mediate protection. We therefore blocked the formation of BALT-like structures by treatment with the soluble decoy receptor lymphotoxin beta-receptor fusion protein (LTβR-Ig). LTβR signaling has been shown to be critical not only for the development of lymphoid organs but also in the maintenance of tertiary lymphoid tissue structures at peripheral sites of inflammation.23, 24, 25 N. brasiliensis–infected mice were treated weekly with the antagonist LTβR-Ig or the isotype control MOPC21 (Figure 4a). Efficiency of treatment was confirmed by the depletion of splenic marginal zone B cells in the LTβR-Ig-treated group (Figure 4b) and abrogated germinal center and marginal zone development in spleens of LTBR-Ig-treated mice as shown previously24, 26 (Figure 4c). Adult worm burdens were quantified at 5 days PI. Equivalent numbers of worms were found in vehicle control and LTBR-Ig-treated mice (Figure 4d). These results indicate that tertiary lymphoid structures are not required for lung-resident T-cell-mediated immunity to N. brasiliensis reinfection.
Lung-resident T cells are independent of tertiary lymphoid structures. Secondary Nippostrongylus brasiliensis (NB)-infected mice were treated weekly with 100 μg MOPC21 isotype or LTβR-Ig (lymphotoxin beta-receptor fusion protein) beginning 3 days before primary infection. (a) Primary infections were cleared at day 7 by Ivermectin treatment, and mice were subsequently reinfected at day 28. (b) Efficiency of the treatment was confirmed by flow cytometric analysis of B220+CD23loCD21hi marginal zone B cells. (c) This was further confirmed by staining spleens with either CD3 (blue)/IgD (brown) or peanut agglutinin (PNA) (blue)/IgD (brown) to look at marginal zone B cells and germinal centers, respectively. Arrows indicate location of these structures. (d) Adult worm burdens were quantified at day 5 post secondary infection to evaluate ability to control infection. Numbers in the flow cytograms indicate the percentage of marginal zone B cells cells relative to lymphocytes. Data are representative of one individual experiment. N=6 mice per group. **P<0.01. IP, intraperitoneal; PI, post infection.
Expansion of lung-resident T cells occurs in the absence of T-cell recruitment from lymph node
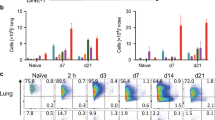
To further define development of N. brasiliensis–responsive lung-resident CD4+ T cells, we compared T-cell immunity in FTY720-treated naive and N. brasiliensis–infected mice (Figure 5a). Goblet cell mucus production, required for parasite expulsion27 was only apparent in infected mice (Figure 5b). Quantification of pulmonary T-cell populations at days 2 and 10 PI showed significantly increased numbers of CD3+CD4+, CD3+CD8+ (Figure 5cA), effector memory and naive CD4+ T cells (Figure 5cB) in infected mice when compared with naive mice. Infected mice showed significantly increased numbers and proportions of lung-resident activated CD4+CD62Llo T cells expressing T1/ST2 (Figure 5cC), indicative of an increased Th2 potential. Re-stimulation of lung cells showed infected mice to secrete higher levels of IL-13 when compared with naive mice (Figure 5d).
Expansion of lung-resident CD4+ T cells occurs in the absence of T-cell recruitment. (a) Naive and Nippostrongylus brasiliensis (NB)-infected mice were treated daily with FTY720 and killed at either 2 or 10 days post infection. (b) Pulmonary tissue was removed, fixed in formalin and stained with periodic-acid Schiff to examine mucus production, × 100 magnification. Single-cell suspensions of whole lung was stained for (cA) CD3+CD4+, CD3+CD8+, (cB) CD4+CD44loCD62Lhi naive, CD4+CD44hiCD62Llo effector memory T cells and (cC) CD3+CD4+CD62LloT1/ST2+ T cells and analyzed by flow cytometry. (d) Whole-cell preparations of lung were re-stimulated with N. brasiliensis excretory secretory proteins for 5 days, and cytokine secretion for interleukin (IL)-13 was detected by enzyme-linked immunosorbent assay. Data are representative of two individual experiment. N=5–6 mice per group. *P<0.05, **P<0.01.
Together, these data suggest that in acutely lymphopenic mice, a pre-existing CD4+ T-cell population in the lung expands in response to N. brasiliensis infection to induce a local and protective Th2 immune response to N. brasiliensis.
Protective immunity to N. brasiliensis reinfection is dependent on T-cell IL-4Rα expression
IL-4Rα signaling is essential for immunity to a range of helminth infections.4, 6, 28, 29 In primary N. brasiliensis infection, worm expulsion does not require IL-4Rα responsiveness by T cells; however, infected CD4+ T-cell IL-4Rα knockout (LckcreIL-4Rα-/lox) mice do have significantly reduced Th2 responses and airway mucus production.20 This indicates that T-cell IL-4Rα responsiveness may contribute significantly to rapid adaptive response, which controls an N. brasiliensis reinfection.
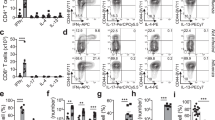
N. brasiliensis reinfection studies comparing primary and secondary infected IL-4Rα-/lox and T-cell-specific IL-4Rα knockout (LckcreIL-4Rα-/lox) mice at 5 days PI (Figure 6a) demonstrated that effective control of secondary N. brasiliensis infection required expression of IL-4Rα on T cells. Here secondary infected LckcreIL-4Rα-/lox mice showed significantly increased intestinal worm burden at day 5 PI when compared with IL-4Rα-/lox control mice (Figure 6b). This reduced protection was associated with decreased numbers of pulmonary T-cell populations (Figure 6c) and IL-13 cytokine production in the lung (Figure 6d). These data further support a role for Th2 CD4+ T cells in coordinating optimal host immunity to N. brasiliensis.
T-cell interleukin (IL)-4Rα expression is required for optimal immunity to Nippostrongylus brasiliensis (NB) reinfection. IL-4Rα-/loxp and LckcreIL-4Rα-/loxp were given a primary (1°) or secondary (2°) N. brasiliensis infection. (a) Primary infections were cleared at day 7 by Ivermectin treatment, and mice were subsequently reinfected at day 28 and killed at 5 days post infection (PI). (b) Intestinal worm burdens were assessed to determine protection kinetics. (c) CD3+CD4+, CD3+CD8+, CD3+CD4+CD44loCD62Lhi naive and CD3+CD4+CD62LloT1/ST2+ T-cell numbers were quantified by flow cytometric analysis of single-cell suspensions of whole lung. (d) Whole-cell preparations of lung were re-stimulated with N. brasiliensis excretory secretory proteins for 5 days and cytokine secretion for IL-13 was detected by enzyme-linked immunosorbent assay. Data are representative of two individual experiments. N=6 mice per group. *P<0.05, **P<0.01.
Lung-resident T cells require IL-4Rα expression for optimal immunity to N. brasiliensis reinfection
FTY720 treatment of secondary infected IL-4Rα-/lox and LckcreIL-4Rα-/lox mice (Figure 7a) showed that control of secondary infection by the lung-resident T cells was also IL-4Rα dependent. FTY720-treated LckcreIL-4Rα-/lox mice showed significantly increased intestinal worm burdens at day 5 PI when compared with IL-4Rα-/lox control mice (Figure 7b). Although no differences were observed in the proportions of T-cell subsets between the FTY720-treated IL-4Rα-/lox and LckcreIL-4Rα-/lox mice (Figure 7c), reduced protection was associated with a decrease in IL-13 cytokine production in the lung of LckcreIL-4Rα-/lox mice (Figure 7d).
Lung-resident T cells require interleukin (IL)-4Rα expression for optimal immunity to Nippostrongylus brasiliensis (NB) reinfection. IL-4Rα-/loxp and LckcreIL-4Rα-/loxp were treated with FTY720 daily or left untreated. (a) Primary infections were cleared at day 7 by Ivermectin treatment, and mice were given a secondary N. brasiliensis infection at day 28 and killed at 5 days post infection (PI). (b) Intestinal worm burdens were assessed to determine protection kinetics. (c) CD3+CD4+ CD3+CD8+, CD3+CD4+CD44loCD62Lhi naive and CD3+CD4+CD62LloT1/ST2+ T-cell numbers were quantified by flow cytometric analysis of single-cell suspensions of whole lung. (d) Whole-cell preparations of lung were re-stimulated with N. brasiliensis excretory secretory proteins for 5 days, and cytokine secretion for IL-13 was detected by enzyme-linked immunosorbent assay . Data are representative of two individual experiments. N=6 mice per group. *P<0.05, **P<0.01.
IL-4Rα expressing lung-resident CD4+ T cells enhance protection to N. brasiliensis reinfection
To demonstrate whether lung-resident CD4+ T cells conferred IL-4Rα-dependent protection against N. brasiliensis reinfection, we isolated CD4+ T cells from the lungs of N. brasiliensis–infected IL-4Rα-/lox or LckcreIL-4Rα-/lox mice treated daily with FTY720. Cells were adoptively transferred intranasally into naive BALB/c mice and subsequently infected with N. brasiliensis (Figure 8a). Transfer of primed IL-4Rα-responsive lung-derived CD4+ T cells into naive BALB/c mice resulted in reduced intestinal worm burdens when compared with recipients of lung-derived LckcreIL-4Rα-/lox CD4+ T cells (Figure 8b). These data confirm that lung-derived CD4+ T cells from lymphopenic mice are important for protection and show that IL-4Rα responsiveness by these lung CD4+ T cells significantly contribute to effective recall immunity to N. brasiliensis infection.
Interleukin (IL)-4Rα expressing lung-resident CD4+ T cells enhance protection to Nippostrongylus brasiliensis (NB) infection. (a) CD4+ T cells were isolated from the lungs of FTY720-treated N. brasiliensis–infected IL-4Rα-/lox or LckcreIL-4Rα-/lox mice and intranasally transferred into FTY720-treated naive BALB/c mice. (b) Naive mice were then infected with 500 L3 N. brasiliensis larvae, and worm burdens were quantified at 5 days post infection (PI). Data are representative of two individual experiments. N=4–6 mice per group. **P<0.01.
Discussion
The key finding in this study demonstrates that in acutely lymphopenic mice, a pre-existing CD4+ T-cell population in the lung can expand in response to N. brasiliensis infection. Expansion of this population was associated with induction of a local and protective Th2 immune response to N. brasiliensis infection. Importantly, this indicates that CD4+ T-cell immunity to N. brasiliensis reinfection is independent of CD4+ T-cell recruitment from the lymph node.
Our data supports and builds on previous studies that indicate the importance of peripheral tissue effector T cells in coordinating host pulmonary immunity.12, 30, 31, 32, 33 These cells can mount an effective secondary T-cell response at the site of infection, leading to increased pathogen clearance. For example, in response to BCG (Bacillus Calmette–Guérin) challenge, protective immune responses have been associated with development of a lung-resident multi-functional CD4+ memory T-cell population independent of recruitment from secondary lymphoid tissue.12, 34 Similarly, in experimental respiratory virus infection, virus-specific memory CD4+ and CD8+ T cells that persist in the lung enable control of challenge infections.14, 34, 35 This protective immunity could be associated with effector lymphoid tissue–like structures16 such as BALT, which contain organized B- and T-cell areas and support local lymphocyte proliferation.36, 37 However, T cells have been shown to respond to presented antigen in a lymph node–free environment.38, 39, 40 The use of LTβR-Ig in our study shows that protective immunity was generated in an environment with impaired stromal cell organization of immune cells in the lung. This suggests that lung interstitial–residing T-cell populations were responsible for the protective immunity we demonstrate in this study.
Our study also shows that CD4+ T cells contribute significantly to control of a secondary N. brasiliensis infection. Previous studies have shown that depletion of CD4+ T cells during secondary infection does not impair recall immunity to N. brasiliensis infection.11 However, depletion of CD4+ T cells in both primary and secondary infection does impair recall immunity.8 Taken together, these studies suggest that CD4+ T cells are required for host immune priming after initial infection but not after secondary infection. This suggests that in the initial infection, these resident CD4+ T cells are activating resident effector cells.
The work we present here builds on these findings by demonstrating that IL-4Rα expression on T cells is required for optimal immunity to secondary N. brasiliensis infection. Our adoptive transfer studies clearly confirm that this protection can be driven by lung-resident T-cell population responses to Th2 cytokines. This represents an important expansion in our understanding of cell-specific IL-4Rα contributions to immunity against helminth infection. This is, to the best of our knowledge, the first demonstration of IL-4Rα expression on a hematopoietic cell population contributing to the control of N. brasiliensis infection.
These findings are of importance to our understanding and control of analogous helminth infections, such as hookworms. We suggest that effective control by vaccination may be most effective where the protective challenge manipulates host immunity to the parasite in the lung.
The most striking finding of this study is, however, a redundancy for SLO-derived CD4+ T cells in recall immunity to N. brasiliensis. This finding has important implications for understanding related Th2 pathologies, such as allergic airway disease and other chronic pulmonary diseases. Furthermore, it sets new targets for vaccine-induced immunity by suggesting that adaptive immune responses at peripheral sites of infection are independent of SLO.
METHODS
Animals used
Six-to-ten-week-old mice were obtained from the University of Cape Town specific pathogen-free animal facility. All experimental procedures were approved by the University of Cape Town Animal Ethics Committee. BALB/c background T-cell-specific IL-4Rα-deficient mice (LckcreIL-4Rα-/lox) were generated as previously described, and hemizygous IL-4Rα-/lox mice were used as controls.41
Administration of FTY720
In order to block migration of lymphocytes from the lymph nodes to peripheral sites, mice received daily administration intraperitoneally of 0.5 mg kg−1 of FTY720 (Enzo Life Sciences, Farmingdale, NY) dissolved in 100 μl of sterile water.
Administration of LTβR-Ig/MOPC21
The systemic antagonist LTβR-Ig (murine IgG1 Fc) and the mouse monoclonal IgG1 control MOPC21 were kindly provided by Biogen/Idec (Cambridge, MA). One hundred micrograms of either reagent was injected intraperitoneally once a week.
N. brasiliensis infection
Mice were inoculated subcutaneously with 500 N. brasiliensis L3 larvae suspended in 0.65–0.9% NaCl using a 21-G needle (Braun, Melsungen, Germany). Infections were cleared at day 7 PI with 10 mg ml−1 of anti-helminthic drug Ivermectin in drinking water.
Adult worm burdens were determined by removing the small intestine and exposing the lumen by dissection. The intestines were incubated at 37 °C for 4 h in 0.65% NaCl to allow the worms to migrate out after which the numbers of worms were counted under a dissecting microscope (Nikon Eclipse, Tokyo, Japan).
Lung worm burdens were quantified as previously described8 by finely cutting the lung, placing on sterile gauze, and suspending them in a 50-ml centrifuge tube containing phosphate-buffered saline at 37 °C for at least 3 h. Viable worms that migrated to the bottom of the tube were counted under a dissecting microscope (Nikon Eclipse).
Preparation of single-cell suspension of lung tissue
Whole lung was removed from individual mice, finely cut, and digested in Iscove’s modified Eagle medium (Invitrogen, Carlsbad, CA) containing 50 U ml−1 collagenase type I (Invitrogen) and 13 μg ml−1 DNase (Roche, Carlsbad, CA) at 37 °C for 90 min. Digested lung tissue were pushed through 70- or 100-μm nylon cell strainer (Becton Dickinson, Franklin Lakes, NJ) and subjected to red cell lysis.
Flow cytometry
In all, 1 × 106 single-cell suspensions from individual lungs were stained in MACS buffer with anti-CD3 Alexa 700 (500A2), anti-CD4 PerCP Cy5.5 (RM4-5), anti-CD8a V500 (53-6.7), anti-CD44 FITC (fluorescein isothiocyanate; IM7), anti-CD62LV450 (MEL-14), and anti-T1/ST2 FITC (DJ8) antibodies to stain for T cells. To stain for splenic marginal zone B cells, the antibodies anti-B220 FITC (RA3-6B2), anti-CD19 Percp-Cy5 (ID3), anti-CD21 allophycocyanin (7G6), and anti-CD23 phycoerythrin (B3B4) were used. Anti-FcR (2.4G2) was used to block non-specific binding of Igs to the FCγII/III receptors. Cells were acquired using FORTESSA Flow cytometer (BD Biosciences, St Paul, MN), and the data analyzed using Flowjo software (Tree Star, Inc., Ashland, OR). CD4+ T-cell subset populations were gated as shown (Supplementary Fig 1 online). Antibodies were purchased from BD Pharmingen, San Diego, CA or MD Bioproducts (St Paul, MN).
Enzyme-linked immunosorbent assay analysis
Whole-lung single-cell preparations were re-stimulated with sub-optimal concentration 2 μg ml−1 of CD3 and 10 μg ml−1 of N. brasiliensis excretory secretory protein and incubated for 120 h at 37 °C. Supernatants from lung re-stimulations were used to measure IL-4 and IL-13 cytokine secretion. In all, 96-well flat-bottom plates (Nunc Maxisorp; Thermo Fisher Scientifica, Roskilde, Denmark) were coated overnight at 4 °C with 50 μl of appropriate coating antibody that was diluted in 1 × phosphate-buffered saline. The plates were then washed four times in wash buffer and subsequently blocked with 200 μl blocking buffer overnight at 4 °C. Following this, three-fold dilutions of the samples and standards were prepared in dilution buffer, and the diluted samples and standards were loaded into wells and incubated overnight at 4 °C. The plates were further washed and 50 μl of appropriate biotinylated secondary antibodies diluted in dilution buffer were added and incubated at 37 °C for 3 h. Fifty microliters of Streptavidin-coupled horseradish peroxidase (HRP) (1/5000 dilution) was added after washing the plates and left in the incubator for 1 h at 37 °C. The plates were developed with TMB microwell peroxidase substrate system, and the reaction was stopped with 1 M H3PO4. The plates were read at an absorbance of 450 nm using a VersaMax microplate reader (Molecular Devices Corporation, Sunnyvale, CA). All antibodies were from BD Pharmingen.
Isolation of CD4+ T-cell population and adoptive transfer experiments
Single-cell suspensions of pooled lungs were prepared at day 10 PI and stained with anti-CD3 phycoerythrin-conjugated and anti-CD4 PerCP-Cy5.5-conjugated monoclonal antibody (BD Pharmingen) before they were isolated (>90% purity) using a FACSVantage cell sorter (Becton Dickinson). In all, 2 × 104 CD4+ T cells were then transferred intranasally in to naive BALB/c mice 24 h before N. brasiliensis infection.
Histology
Intestinal and lung tissue were fixed in neutral-buffered formalin solution and embedded in paraffin before cutting in to 5-μm section with a cryostat. Sections were stained with periodic-acid Schiff reagent for quantification of pulmonary and intestinal mucus production.
Spleens frozen in liquid nitrogen were cut at 7-μm thickness and fixed with acetone. Sections were stained with either rat anti-mouse IgM or peanut agglutinin coupled to HRP (B-1075) to delineate marginal zone B cells and germinal centers, respectively. Follicular areas were stained using sheep anti-mouse IgD. Sections were thereafter stained with their respective secondary antibodies, rabbit anti-rat biotin, and donkey anti-sheep HRP. Slides were then washed and streptavidin-complex with alkaline phosphatase was added (Dako, Glostrup, Denmark) for 30 min. Slides were developed sequentially using DAB (3,3′diaminobenzidine) and napthol AS-MX phosphate with Fast Blue salt, respectively. All antibodies were purchased from Abcam (Cambridge, UK).
Confocal microscopy
Lungs were inflated with 1:1 OCT (optimal cutting temperature):phosphate-buffered saline, embedded in OCT, and frozen over liquid nitrogen. Sections were cut at 9-μm thickness, fixed with acetone, air dried, and stained for CD3+ T cells. Rat anti-mouse CD3 (KT3) was purchased from Abcam. Goat anti-rat Alexa-488 was used for detection, and slides were counterstained with Hoechst nuclear stain. All sections were viewed with a Zeiss Axiovert LSM 510 Meta NLO microscope (Zeiss, Jena, Germany).
Statistics
Data were expressed as mean±s.d. and analyzed using one-tailed Mann–Whitney nonparametric T test with a 95% confidence interval. P-value <0.05 were considered significant and are indicated by an asterisk.
References
Hotez, P.J., Brindley, P.J., Bethony, J.M., King, C.H., Pearce, E.J. & Jacobson, J. Helminth infections: the great neglected tropical diseases. J. Clin. Invest. 118, 1311–1321 (2008).
Horsnell, W.G.C. et al. Delayed goblet cell hyperplasia, acetylcholine receptor expression, and worm expulsion in SMC-specific IL-4Ralpha-deficient mice. PLoS pathog. 3, e1 (2007).
Kopf, M., Le Gros, G., Bachmann, M., Lamers, M.C., Bluethmann, H. & Köhler, G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature 362, 245–248 (1993).
Brombacher, F. The role of interleukin-13 in infectious diseases and allergy. BioEssays. 22, 646–656 (2000).
Anthony, R.M., Rutitzky, L.I., Urban, J.F. Jr. & Stadecker, M.J. Protective immune mechanisms in helminth infection. Nat. Rev. Immunol. 7, 975–987 (2007).
Urban, J.F. Jr., Noben-Trauth, N., Schopf, L., Madden, K.B. & Finkelman, F.D. Cutting edge: IL-4 receptor expression by non-bone marrow-derived cells is required to expel gastrointestinal nematode parasites. J. Immunol. 167, 6078–6081 (2001).
Horsnell, W.G.C. et al. IL-4Rα-responsive smooth muscle cells contribute to initiation of TH2 immunity and pulmonary pathology in Nippostrongylus brasiliensis infections. Mucosal. Immunol. 4, 83–92 (2011).
Harvie, M., Camberis, M., Tang, S.-C., Delahunt, B., Paul, W. & Le Gros, G. The lung is an important site for priming CD4 T-cell-mediated protective immunity against gastrointestinal helminth parasites. Infect. Immun. 78, 3753–3762 (2010).
Anthony, R.M. et al. Memory TH2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat. Med. 12, 955–960 (2007).
Sallusto, F., Geginat, J. & Lanzavecchia, A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22, 745–763 (2004).
Katona, I.M., Urban, J.F. Jr. & Finkelman, F. The role of L3T4+ and LYT-2+ T cells in the IgE response and immunity to Nippostrongylus brasiliensis. J. Immunol. 140, 3206–3211 (1988).
Connor, L.M. et al. A key role for lung-resident memory lymphocytes in protective immune responses after BCG vaccination. Eur. J. Immunol. 40, 2482–2492 (2010).
Harris, N.L., Watt, V., Ronchese, F. & Le Gros, G. Differential T cell function and fate in lymph node and nonlymphoid tissues. J. Exp. Med. 195, 317–326 (2002).
Hogan, B.R.J., Zhong, W., Usherwood, E.J., Cookenham, T., Roberts, A.D. & Woodland, D.L. Protection from respiratory virus infections can be mediated by antigen-specific CD4 T Cells that persist in the lungs. J. Exp. Med. 193, 0–5 (2001).
Teijaro, J.R., Turner, D., Pham, Q., Wherry, E.J., Lefrançois, L. & Farber, D.L. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol. 187, 5510–5514 (2011).
Zaph, C., Rook, K.A., Goldschmidt, M., Mohrs, M., Scott, P. & Artis, D. Persistence and function of central and effector memory CD4+ T cells following infection with a gastrointestinal helminth. J. Immunol. 177, 511–518 (2006).
van Panhuys, N., Perret, R., Prout, M., Ronchese, F. & Le Gros, G. Effector lymphoid tissue and its crucial role in protective immunity. Trends Immunol. 26, 242–247 (2005).
Chiba, K., Matsuyuki, H., Maeda, Y. & Sugahara, K. Role of sphingosine 1-phosphate receptor type 1 in lymphocyte egress from secondary lymphoid tissues and thymus. Cell. Mol. Immunol. 3, 11–19 (2006).
Hofmann, M., Brinkmann, V. & Zerwes, H.-G. FTY720 preferentially depletes naive T cells from peripheral and lymphoid organs. Int. Immunopharmacol. 6, 1902–1910 (2006).
Mearns, H. et al. Interleukin-4-promoted T helper 2 responses enhance Nippostrongylus brasiliensis-induced pulmonary pathology. Infect. Immun. 76, 5535–5542 (2008).
Lohning, M. et al. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc. Natl. Acad. Sci. USA 95, 6930–6935 (1998).
Sullivan, B.M. et al. Genetic analysis of basophil function in vivo. Nat. Immunol. 12, 527–535 (2011).
Browning, J.L. Inhibition of the lymphotoxin pathway as a therapy for autoimmune disease. Immunol. Rev. 223, 202–220 (2008).
Fava, R.A. et al. A role for the lymphotoxin/LIGHT axis in the pathogenesis of murine collagen-induced arthritis. J Immunol. 171, 115–126 (2003).
van de Pavert, S.A. & Mebius, R.E. New insights into the development of lymphoid tissues. Nat. Rev. Immunol. 10, 664–674 (2010).
Fava, R. et al. Lymphotoxin-beta receptor blockade reduces CXCL13 in lacrimal glands and improves corneal integrity in the NOD model of sjogren’s syndrome. Arthritis Res. & Ther. 13, R182 (2011).
Hasnain, S.Z. et al. Muc5ac: a critical component mediating the rejection of enteric nematodes. J. Exp. Med. 208, 893–900 (2011).
Barner, M., Mohrs, M., Brombacher, F. & Kopf, M. Differences between IL-4R alpha-deficient and IL-4-deficient mice reveal a role for IL-13 in the regulation of Th2 responses. Curr. Biol. 8, 669–672 (1998).
Herbert, D.R. et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity 20, 623–635 (2004).
Chapman, T.J., Lambert, K. & Topham, D.J. Rapid reactivation of extralymphoid CD4 T cells during secondary infection. PloS One 6, e20493 (2011).
Masopust, D., Vezys, V., Marzo, A.L. & Lefrançois, L. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291, 2413–2417 (2001).
Moyron-Quiroz, J.E. et al. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity 25, 643–654 (2006).
Hogan, R.J. et al. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J. Immunol. 166, 1813–1822 (2001).
Kaveh, D.A., Bachy, V.S., Hewinson, R.G. & Hogarth, P.J. Systemic BCG immunization induces persistent lung mucosal multifunctional CD4 T(EM) cells which expand following virulent mycobacterial challenge. PloS One 6, e21566 (2011).
Hogan, R.J. et al. Long-term maintenance of virus-specific effector memory CD8+ T cells in the lung airways depends on proliferation. J. Immunol. 169, 4976–4981 (2002).
Moyron-Quiroz, J.E. et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat. Med. 10, 927–934 (2004).
Randall, T.D. Bronchus-associated lymphoid tissue (BALT) structure and function. Adv. Immunol. 107, 187–241 (2010).
Hofmann, J., Greter, M., Du Pasquier, L. & Becher, B. B-cells need a proper house, whereas T-cells are happy in a cave; the dependence of lymphocytes on secondary lymphoid tissues during evolution. Trends Immunol. 31, 144–153 (2010).
Wakim, L.M., Waithman, J., van Rooijen, N., Heath, W.R. & Carbone, F.R. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science 319, 198–202 (2008).
Constant, S.L. et al. Resident lung antigen-presenting cells have the capacity to promote Th2 T cell differentiation in situ. J. Clin. Invest. 110, 1441–1448 (2002).
Dewals, B. et al. IL-4Ralpha responsiveness of non-CD4 T cells contributes to resistance in Schistosoma mansoni infection in pan-T cell-specific IL-4Ralpha-deficient mice. Am. J. Pathol. 175, 706–716 (2009).
Acknowledgements
We thank Biogen Idec for supplying us with LTβR-Ig and MOPC21 reagents, Rayaana Fredericks and Wendy Green for their technical assistance, Rodney Lucas for assisting in animal procedures, Lizette Fick and Dunja Mrdjen for their assistance with histology, and Dr Dirk Lang and Susan Cooper for assisting with confocal microscopy. The work presented was funded by National Research Foundation, Medical Research Council (South Africa), International Centre for Genetic Engineering and Biotechnology, Worldwide Universities Network, and Claude Leon Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Rights and permissions
About this article
Cite this article
Thawer, S., Horsnell, W., Darby, M. et al. Lung-resident CD4+ T cells are sufficient for IL-4Rα-dependent recall immunity to Nippostrongylus brasiliensis infection. Mucosal Immunol 7, 239–248 (2014). https://doi.org/10.1038/mi.2013.40
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2013.40
This article is cited by
-
Tissue resident memory T cells in the respiratory tract
Mucosal Immunology (2022)
-
Regulatory role of Gpr84 in the switch of alveolar macrophages from CD11blo to CD11bhi status during lung injury process
Mucosal Immunology (2020)
-
Intestinal helminth infection promotes IL-5- and CD4+ T cell-dependent immunity in the lung against migrating parasites
Mucosal Immunology (2019)
-
Helminth-induced IL-4 expands bystander memory CD8+ T cells for early control of viral infection
Nature Communications (2018)
-
Regionally compartmentalized resident memory T cells mediate naturally acquired protection against pneumococcal pneumonia
Mucosal Immunology (2018)