Abstract
The ability of the colon to generate an immune response to pathogens, such as the model pathogen Trichuris muris, is a fundamental and critical defense mechanism. Resistance to T. muris infection is associated with the rapid recruitment of dendritic cells (DCs) to the colonic epithelium via epithelial chemokine production. However, the epithelial–pathogen interactions that drive chemokine production are not known. We addressed the role of the cytosolic pattern recognition receptor Nod2. In response to infection, there was a rapid influx of CD103+CD11c+ DCs into the colonic epithelium in wild-type (WT) mice, whereas this was absent in Nod2−/− animals. In vitro chemotaxis assays and in vivo experiments using bone marrow chimeras of WT mice reconstituted with Nod2−/− bone marrow and infected with T. muris demonstrated that the migratory function of Nod2−/− DCs was normal. Investigation of colonic epithelial cell (CEC) innate responses revealed a significant reduction in epithelial production of the chemokines CCL2 and CCL5 but not CCL20 by Nod2-deficient CECs. Collectively, these data demonstrate the importance of Nod2 in CEC responses to infection and the requirement for functional Nod2 in initiating host epithelial chemokine-mediated responses and subsequent DC recruitment and T-cell responses following infection.
Similar content being viewed by others
INTRODUCTION
The gastrointestinal-dwelling parasite, Trichuris muris (T. muris), is a natural infection of mice and is also used as a model for the human parasite Trichuris trichiura (T. trichiura). T. trichiura infection affects over 1 billion people, with the highest prevalence being in developing countries.1 Patients have a spectral immune response, with some people immune to infection despite being in a disease endemic country, whereas others are susceptible and harbor long-term chronic infection.2 This spectral immune response is also reflected in T. muris infection in mice. A strong T helper type 2 (Th2) response governs immunity to the parasite, whereas a dominant T helper type 1 (Th1) response renders the host susceptible to infection.2 Although the immune responses that govern resistance and susceptibility to T. muris are well characterized, how and why these immune responses are initiated is still unclear. Dendritic cells (DCs) are important cells for priming T cells and driving T-cell subset polarization.3 Epithelial cells have been shown to play a critical role in promoting this ability of DCs to polarize T-cell responses,4, 5 and therefore the epithelial/DC interaction may be an underlying factor as to why we observe differing immune responses to T. muris and indeed T. trichiura. T. muris is known to burrow into the epithelium of the large intestine and remains throughout its lifetime with its head end buried within an epithelial syncytial tunnel.2 Given the close proximity of T. muris within the epithelial layer of the colon, it is feasible that epithelial cells sense and respond to the parasite and initiate DC priming and immunity. Indeed, previous work has shown that immortalized intestinal epithelial cells are able to respond to T. muris antigen,6 and work from our group demonstrated that resistance to infection is associated with the rapid recruitment of DCs to the colonic epithelium via epithelial chemokine production.7 However, the epithelial–parasite interaction that drives chemokine production and therefore DC recruitment is not known. Rapid recruitment of DCs to the colonic epithelium in T. muris infection was also associated with accelerated maturation of DCs,7 thus implying that DC recruitment to the epithelium is necessary for epithelial conditioning of DCs and induction of Th2-driven immunity.
Epithelial cells express several evolutionarily conserved and structurally related proteins called pattern recognition receptors (PRRs) that recognize specific microbe-associated molecular patterns such as lipopolysaccharide (LPS) or peptidoglycan that are found on the surface of pathogens. PRRs also detect damage-associated molecular patterns associated with tissue injury or cell death caused by inflammation and infection.8 One such family of PRRs is the Nod-like receptors (NLRs) that are primarily intracellular PRRs located within the cytosol of cells.9 The NLR Nod2 is a PRR of interest as mutations in the Nod2 gene have been associated with the inflammatory disorder Crohn’s disease, as well as increased susceptibility to infections.9 The highest levels of Nod2 expression are found in epithelial cells and antigen-presenting cells,10, 11 although Nod2 has also been identified in T cells12 and neutrophils.13 Within the colonic epithelium, Nod2 expression is thought to be restricted to the dividing cells at the base of the crypts14 that corresponds to the T. muris niche during the early phase of infection.2 Nod2 specificity was originally thought to be restricted to the detection of muramyl dipeptide (MDP) on Gram-positive and Gram-negative bacteria, although now it is known to have a diverse role in host immunity. Nod2 has been attributed in virus recognition,15 T-cell signalling,16 adaptive immune responses,17 and the regulation of host–microbiota crosstalk.18, 19 However, a role for Nod2 in helminth immunity is yet to be defined. In this study we investigated the role of Nod2 in the initiation of the immune response to T. muris. We found that Nod2−/− mice had a reduced CD103+ DC recruitment to the epithelium. Furthermore, Nod2−/− mice had delayed parasite expulsion kinetics. Impaired DC recruitment was attributed to a reduction in colonic epithelial cell (CEC) responsiveness and chemokine secretion in response to infection. Our data implicate a role for Nod2 in the initiation of immunity to infection via the regulation of CEC-derived chemokines and subsequent DC recruitment.
RESULTS
Upregulation of Nod2 expression by CECs in response to T. muris infection
T. muris penetrates the colonic epithelial layer with its head remaining buried within the epithelium for the bulk of its life cycle. Therefore, we investigated the upregulation of Nod2 and Rip2 in CECs in early T. muris infection in C57BL/6 wild-type (WT) mice by quantitative PCR (qPCR). Modulation of Nod2 expression by the parasite was seen within 24 h of infection, with an approximately two-fold increase in the expression of Nod2 in CECs of C57BL/6 mice (P<0.001, Figure 1a) at 24 h after infection. Expression of Rip2 was also increased approximately twofold in CECs at 24 h after infection (Figure 1b).
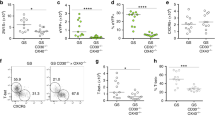
Impaired recruitment of CD103+ dendritic cells (DCs) to the colonic epithelium in Nod2−/− mice in response to Trichuris muris. Wild-type (WT) and Nod2−/−mice were infected orally with ∼175 embryonated T. muris eggs. Colonic epithelial cells from WT mice were analyzed before and after infection by quantitative PCR (qPCR) for (a) Nod2 and (b) Rip2 mRNA; n=3–12. Data for time points D0 and D1 are pooled from two individual experiments. Lamina propria and intraepithelial cells were isolated from the large intestine and stained for CD45, MHC-II, CD11c, CD103, and F4/80 on D0, D1, D2, D5, D7, and D9 after infection. (c) Gating strategy for CD103+ and F4/80-positive cells isolated from the large intestine. (d) Percentage of MHCII+CD11c+CD103+F4/80− DCs were calculated as a percentage of the CD45+ cell population. (e) Percentage of MHCII+CD11c+CD103− F4/80+ macrophages were calculated as a percentage of the CD45+ cell population. Data are representative of at least 3–4 mice each from 3 experiments (total n=9–12) with the exception of D7 and D9 that are representative of 1 experiment (n=4). (f) Mice infected with T. muris were injected with 5-bromodeoxyuridine (BrdU) 16 h before killing. Lamina propria and intraepithelial cells were isolated from the large intestine and stained for CD45, MHCII, CD11c, CD103, and BrdU, and the number of BRDU+CD103+ of CD45+ population quantified (n=5; *P<0.05, **P<0.001).
Nod2−/− mice have delayed recruitment of CD103+ DCs to the large-intestinal epithelium
Rapid DC recruitment to the colon has been shown to be associated with resistance to T. muris.7 To assess whether Nod2 plays a role in recruitment of DCs in T. muris infection, we analyzed the recruitment of macrophages and DCs to the large intestine in response to T. muris infection (Figure 1c–e). There was no difference between the proportion of CD103+ DCs (CD11c+ MHCIIhi) in the large intestine of naive C57BL/6 WT and Nod2−/− animals (Figure 1d). At day 1 (D1) after infection, we observed an increase in the proportion of CD103+ DCs (CD11c+ MHCIIhi) recruited to the large intestine in C57BL/6 WT animals but not in Nod2−/− mice (Figure 1d, P=0.02). Although proportions of CD103+ DCs between C57BL/6 WT and Nod2−/− mice were similar at D2, the difference in magnitude of the DC response between Nod2−/− and C57BL/6 WT mice was more dramatic at day 5 (D5) after infection (P=0.01), with C57BL/6 mice showing a threefold increase in the proportion of large-intestinal DCs (∼17% of the CD45+ population) compared with Nod2−/− animals in which there was only a modest increase (∼5% of the CD45+ population). The percentage of DCs remained higher in C57BL/6 mice at days 7 and 9 after infection (Figure 1d) compared with Nod2−/− mice. We observed no difference in the intensity of expression of CD103, suggesting there was no upregulation of CD103 expression after infection (data not shown). In contrast, with regard to recruitment of CD103+ DCs, no difference was observed in the proportion of CD103− negative colonic DC populations between Nod2−/− and C57BL/6 WT at all time points analyzed (D1: Nod2−/− 2.00±0.88%, C56BL/6 3.72% ±1.5; D2: Nod2−/− 1.37±0.08%, C56BL/6 1.45% ±0.78; D5: Nod2−/− 7.64±1.25%, C57BL/6 6.5% ±1.6; D7–D9: data not shown). Overall, the magnitude of CD103+ DC recruitment in Nod2−/− animals in response to infection was reduced at all time points studied.
To see whether there was a general reduction in the phagocyte response to T. muris in Nod2−/− mice, we also assessed macrophages (F4/80+MHCII+) (Figure 1e). In contrast to the increased proportions of DCs in WT but not Nod2−/− mice after infection, there was no difference in the proportion of large-intestinal macrophages between Nod2−/− and C57BL/6 mice before or after infection at all time points studied (Figure 1e). Interestingly, the peak of DC recruitment at D5 after infection seen in WT mice also corresponded to a small peak in macrophage recruitment, although there was no difference between WT and Nod2−/− mice with both strains having an equivalent response. To see whether the changes in the proportions of DCs/macrophages after infection between WT and Nod2−/− mice were restricted to the large intestine, we also investigated macrophage and DCs proportion in the mesenteric lymph node (MLN) and spleen and saw no differences.
Immunohistochemistry analysis was used to validate the flow cytometry findings and define phagocyte localization before and after infection. Results showed higher frequencies of CD11c+ cells in the colon and cecum of C57BL/6 mice compared with Nod2−/− mice (Figure 2a,b). As CD11c alone is not a discrete marker of DCs, we performed further validation using antibodies against CD103, CD11b, or F4/80, and counted the number of CD103+ CD11c+ CD11b+ cells and F480+CD11c+ cells (Figure 2c–f). Our data showed an increase in the number of CD11c+CD103+ cells in C57BL/6 WT mice (Figure 2c,d, P=0.04) and verified the reduced number of CD103+CD11c+ DCs in the large intestine of Nod2−/− animals (Figure 2). The majority of DCs in both WT and Nod2−/− animals were CD11b+ in early infection with no significant differences in the proportions of CD11b+ DCs between strains or after infection (83.%±15.8 in naive WT mice vs. 84.29±7.37% in naive Nod2−/− mice; 87.5±7.98% in WT vs. 95±5% in Nod2−/− mice at D1 after infection; 96.67±28% in WT mice vs. 91.89±8.1% in Nod2−/− mice at D5 after infection, Supplementary Figure S1 online and data not shown). DC localization changed during T. muris infection. Within the large intestine, DCs are scarce, located deep within the lamina propria far away from the epithelium (Figure 2a). Upon T. muris infection within the WT mice, DCs were observed higher up the crypt axis close or adjacent to epithelial cells, whereas this was not observed in Nod2−/− mice (Figure 2). The numbers of F480+CD11c+ cells were more variable after infection but overall showed little difference in the number or distribution between Nod2−/− and WT mice, with most macrophages restricted to the lamina propria below the crypts (Figure 2e,f).
Impaired recruitment of CD103+ dendritic cells (DCs) to the colonic epithelium in Nod2−/− mice in response to Trichuris muris. Frozen cecal and colon sections were taken at autopsy, sectioned, and stained for nuclei (blue), cytokeratin (green), and CD11c (red). (a) Representative images are shown (scale bar for naives=100 μm; day 2 (D2) after infection=50 μm). (b) Quantification of CD11c+ cells in Nod2−/− and C57BL/6 mice. Frozen cecal and colon sections were taken at autopsy, sectioned, and stained for nuclei (4’,6-diamidino-2-phenylindole (DAPI), blue), CD11c (red), CD103, or F4/80 (green). Colocalized cells are shown in yellow. (c) Representative image of CD103 staining (scale bar=50 μm (insert=30 μm)). (d) Quantification of dual-stained CD103+ CD11c+ cells. (e) Representative image of F4/80 staining (scale bar=50 μm (insert=40 μm)). (f) Quantification of dual-stained F4/80 CD11c cells; n=3 (C57BL/6) and n=4 (Nod2−/−) per time point. Data shown are mean +/−s.e.m. *P=0.04.
Increased CD103+ numbers is due to migration not proliferation
To assess whether the increased proportion of CD103+ DCs observed in the colon of C57BL/6 WT mice was because of in situ proliferation or migration of the cells to the site of infection, we assessed 5-bromodeoxyuridine (BrdU) uptake in DCs in the colon of Nod2−/− and C57BL/6 WT animals after infection by flow cytometry. At D5 after infection, low levels of CD103+ BrdU+ dual-positive cells were observed in C57BL/6 and Nod2−/− animals at equivalent levels (Figure 1f). These data indicate that the increased proportion of DCs observed in C57BL/6 WT mice compared with Nod2−/− is not due to in situ proliferation and more likely because of altered DC recruitment into the large intestine.
Impaired T. muris expulsion kinetics in Nod2−/− mice
To assess whether the delayed recruitment of CD103+ DCs affected parasite expulsion, Nod2−/− and C57BL/6 mice were infected with T. muris and worm burdens assessed at D21. C57BL/6 mice are resistant to infection and most mice expel their worms by day 21 after infection. However, whereas most of the C57Bl/6 mice had expelled their worms, Nod2−/− animals had not and there was a significantly higher worm burden in Nod2−/− animals compared with C57BL/6 WT mice (Figure 3a, P=0.006). In addition, we measured interferon-γ and interleukin-13 (IL-13) levels from MLN cells restimulated with T. muris excretory/secretory (E/S) antigens at day 21 after infection, at the peak cytokine response. Both strains of mice produced interferon-γ and IL-13 that is typical of the mixed Th1/Th2 response observed in C57BL/6 mice.2 Levels of interferon-γ and IL-13 were similar between Nod2−/− and C57BL/6 WT mice (Figure 3b,c). In addition, we observed no difference in the levels of IL-4, IL-6, IL-9, tumor necrosis factor-α, and IL-12p70 observed in Nod2−/− and C57BL/6 mice. However, as both strains of mice had expelled some worms by D21 after infection, we looked at the early T-cell response (D7, D10, and D18 after infection) during T. muris infection in Nod2−/− mice to see whether the early dynamics of the T-cell response were altered. Analysis of T cells in the MLNs revealed a reduction in both CD4 and CD8 T cells in Nod2−/− mice early after infection (Figure 3d,e). We then further analyzed T cells positive for the gut-homing marker CCR9 and saw that both CD4+ CCR9+ (Figure 3f) and activated/ memory cells CD44hi CD62Llo (Figure 3g) were significantly reduced at D18 in the MLNs of Nod2−/− mice. Collectively, these data suggest that the reduced DC response in Nod2−/− mice impairs the early adaptive immune response to T. muris.
Delayed expulsion kinetics of Trichuris muris in Nod2−/− mice. (a) Mice were infected orally with ∼175 embryonated T. muris eggs and worm burdens were assessed at day 21 (D21) after infection. Data shown are for individual mice with mean values per group. Data are representative of two independent experiments with n=4 (Nod2−/−) and n=5 (C57BL/6) in each experiment. (b) Mesenteric lymph node (MLN) cells were restimulated with excretory/secretory (E/S) at day 21 after infection. (b) Interleukin-13 (IL-13) and (c) interferon-γ (IFN-γ) levels in supernatants were then assayed by cytokine bead array. MLN cells were harvested from naive mice at D7, D10, and D18 post-infection, (d, e) the numbers of T cells were enumerated, and (f) the numbers of gut-homing (CCR9+) CD4 T cells and activated/memory CD62LloCD44hi cells (g) were enumerated by flow cytometry. Analyses of data are representative of two independent experiments each with n=2–4 (naive animals) and n=5 (infected animals). *P<0.05; NS, not significant.
Nod2 does not drive basophil recruitment
Basophils have been implicated in mediating Th2 immunity against T. muris.20 Furthermore, DCs alone were shown to be insufficient at mediating immunity to T. muris.20 We therefore assessed basophil frequencies in C57BL/6 and Nod2−/− after infection with T. muris (Figure 4). At D5 after infection, there was no difference in the frequency of basophils in C57BL/6 and Nod2−/− mice, suggesting no role for Nod2 in the recruitment of basophils in T. muris infection (Figure 4a,b).
Increased CD103+ cells in the large intestine is not due to in situ proliferation of dendritic cells (DCs), and Nod2 has no role in basophil recruitment to the large intestine. Mice were infected orally with ∼175 embryonated Trichuris muris eggs. Lamina propria and intraepithelial cells were isolated from the large intestine and stained for lineage markers (CD4, CD8α, B220, CD19), c-kit, and FcεR. (a) Gating strategy for basophils (Lin− C-kit− FcεR+) cells. (b) Quantification of Lin− c-kit− FcεR+ cells in the large intestine and mesenteric lymph nodes (MLNs); n=5 (C57BL/6) and n=4 (Nod2−/−).
Nod2−/− DCs can migrate normally in vivo
Nod2 is expressed by intestinal epithelial cells and immune cells, and therefore we addressed whether the impaired recruitment of DCs was because of an inability of Nod2−/− DCs to migrate to the epithelium or because of impaired responses by Nod2−/− epithelial cells. Chemokines are key drivers in the migration of immune cells to sites of infection. We therefore first assessed the level of expression of the chemokine receptors CCR5 and CCR2 on DCs by flow cytometry in naive and infected mice and showed that the levels of chemokine receptors were equivalent (Figure 5a,b). These data imply that Nod2−/− DCs have a similar potential and ability to respond to chemokines as WT DCs. Indeed, we performed further analysis of DC migratory function by in vitro chemotaxis assay (Figure 5c). Our data show that equivalent numbers of colonic DCs from Nod2−/− and C57BL/6 were able to migrate toward CCL5 and CCL2. In the event that there could be a defective response of Nod2−/− DCs to parasite, we also investigated the response of DCs to T. muris antigen. T. muris resides within an epithelial syncytial tunnel and does not breach the epithelial layer, and therefore we investigated the interaction of DCs with parasite secretory antigen that may cross the epithelial barrier. Our data show that Nod2−/− DCs upregulated major histocompatibility complex-II (MHC-II; Figure 5d), CD86, and CD80 (data not shown) at equivalent levels to C57BL/6 WT animals in response to LPS or T. muris antigen. Combined, these data suggest that Nod2−/− DC migratory function and ability to respond to T. muris is normal.
Nod2−/− dendritic cells (DCs) express chemokine receptors and can migrate effectively in vitro. Mice were infected orally with ∼175 embryonated Trichuris muris eggs. Lamina propria and intraepithelial cells were isolated from the large intestine DCs isolated from the large intestine were stained with chemokine receptors (a) CCR5 and (b) CCR2. (c) A chemotaxis assay was performed using colonic DCs and CCL2 (10 ng ml−1) and CCL5 (1 ng ml−1); n=5 (C57BL/6) and n=3–4 (Nod2−/−). (d) Bone marrow–derived DCs (BMDCs) were cultured with medium (negative control), lipopolysaccharide (LPS; 5 μg ml−1), or T. muris antigen (T. muris Ag; 5 μg ml−1) for 24 h before being stained for CD45, CD11c, and major histocompatibility complex-II (MHC-II) and analyzed by flow cytometry. Data shown are the average of three replicates and are representative of two experiments.
We next assessed the ability of Nod2−/− DCs to migrate to Nod2+/+ epithelium in vivo by generating bone marrow chimeras. C57BL/6 WT mice irradiated and reconstituted with Nod2−/− bone marrow (herein referred to as C57BL/6nod2−/− (Figure 6a) were used to assess the responses of Nod2-deficient DCs to Nod2-sufficient CECs in response to T. muris infection. Successful reconstitution of C57BL/6 WT with Nod2−/− bone marrow was assessed by determining Nod2 ligand responsiveness of isolated DCs (Figure 6b). As expected, bone marrow DCs derived from C57BL/6nod2−/− were unresponsive to the Nod2 ligand (P=0.01), MDP, but were responsive to LPS, identifying the bone marrow cells as being of Nod2−/− origin and that chimerism had been achieved. Colonic cells from the chimeras at D5 after infection were assessed for the presence of CD103+ DCs. In C57BL/6nod2−/− CD103+ DCs had migrated to the colonic epithelium, with the proportion of DCs being equivalent to that of normal C57BL/6 mice (Figure 6c). We did attempt to perform a reverse bone marrow chimera and reconstituted Nod2−/− mice with WT bone marrow (Nod2wt). When we irradiated Nod2-deficient mice, the majority of the mice died within a few days after irradiation that we attributed to a defect in epithelial homeostasis as we published previously.14 However, of the mice that survived, we were able to confirm a defect in immune cell recruitment to T. muris infection (Figure 6d, P=0.01) and DC recruitment with only 7.1±1.1 CD103+CD11c+ DCs in the lamina propria of Nod2wt chimeras compared with 17.75+3.2 DCs in WTnod2 chimeras (Figure 6e, P=0.01). Collectively, these data demonstrate that Nod2−/− DCs are able to migrate effectively in vivo in response to T. muris.
Nod2−/− dendritic cells (DCs) can migrate to a Nod2+/+ epithelium. (a) A schematic representation of generation of bone marrow chimeras. (b) Successful reconstitution was determined by harvesting bone marrow from C57BL/6Nod−/− mice and stimulating with medium (negative control), muramyl dipeptide (MDP; 1.0 μg ml−1), and lipopolysaccharide (LPS; 100 ng ml−1, positive control). DC responses to the various ligands were analyzed by assessing the level of maturation by CD86, CD40, and major histocompatibility complex-II (MHC-II) upregulation by flow cytometry. The graphs show the percentage of MHCIIhiCD40+ CD86+ CD11c cells in each culture condition. (c) Lamina propria and intraepithelial cells were isolated from the large intestine of C57BL/6Nod−/− and C57BL/6wt mice and stained for CD45, MHC-II, CD11c, CD103, and F4/80 on day 5 (D5) after infection and measured as a percentage of the CD45+ population (n=4–5). (d) A defect in CD45 cell recruitment was also observed in Nod2wt mice compared with controls. (e) Immunohistochemistry staining revealed a defect in CD11c+CD103+ DC recruitment in Nod2wt mice compared with C57BL/6Nod−/− mice. *P=0.01.
Nod2−/− epithelial cells have impaired chemokine secretion
Previous data from our group showed a role for epithelial cell-derived chemokines in DC recruitment to T. muris.7 It is therefore possible that the impaired recruitment of CD103+ DCs in Nod2−/− mice was because of defective epithelial responses and production of chemokines. To assess CEC function in Nod2−/− mice, CECs were harvested from the epithelium of C57BL/6 WT and Nod2−/− mice and cultured in vitro with T. muris E/S antigen, T. muris eggs, and a medium control using our validated isolation and culture methods and the supernatants analyzed by enzyme-linked immunosorbent assay (ELISA; Figure 7 and data not shown). Cultured CECs from C57BL/6 WT animals constitutively produced CCL2, CCL5, and CCL20 (Figure 7a–d). In contrast, CECs from Nod2−/− mice produced significantly lower amounts of CCL2 (P<0.001) and CCL5 (P<0.05, Figure 7a,b). There was no significant reduction in CCL20 secretion from Nod2−/− CECs compared with WT CECs (Figure 7c). Importantly, Nod2−/− CECs were able to secrete chemokines, although at a reduced magnitude as compared with WT CECs. Indeed, levels of secretion of the alarmin IL-33 showed that secretion was equivalent in WT and Nod2−/− CECs (Figure 7d). Addition of E/S antigen or T. muris eggs did not increase chemokine secretion from C57BL/6 WT or Nod2−/− CEC (data not shown), suggesting that epithelial cells were not responding to either eggs or parasite secretory antigen. As we saw a significant difference in the levels of CCL3 and CCL5 by ELISA, we also investigated levels of these chemokines in epithelial cells in vivo by qPCR. The qPCR data showed a trend for reduced expression of CCL5, CCl2, and IL33 in Nod2−/− CEC after infection, although this was not significant (Figure 7e–g).
Nod2−/− colonic epithelial cells are unable to produce the chemokines CCL2, CCL5, and CCL20. (a–d) Colonic epithelial cells were cultured from C57BL/6 and Nod2−/− mice and the levels of CCL2, CCL5, CCL20, and interleukin-33 (IL-33) were measured by enzyme-linked immunosorbent assay (ELISA) (n=8 and data are representative of two independent experiments). (e–g) Colonic epithelial cells were harvested from the colon of naive and infected C57BL/6 and Nod2−/− mice on day 1 (D1) after infection. The mRNA levels of CCL5, CCL2, and IL-33 were measured by quantitative PCR (qPCR) and data show relative expression (n=11–13 and are pooled from two individual experiments). *P<0.05; **P<0.001.
DISCUSSION
The immune response to T. muris is well characterized, with resistance being associated with an archetypal Th2 response and susceptibility with a dominant Th1 response.21 However, the mechanisms that govern the initiation of the immune response, in terms of the recognition of infection, and recruitment of immune cells to the site of infection, are unclear. The findings from our study document a role for Nod2 in CECs in the production of chemokines that drive CD103+ DC recruitment to the epithelium in response to T. muris infection. These findings shed new light on the role of PRRs and Nod2 in initiating host responses to invading pathogens in the colon.
Nod2 has been previously shown to be important in the production of chemokines. Consistent with our findings, a recent study showed that Nod2−/− mice had impaired secretion of CCL2 from the stromal compartment of the small intestine in response to bacterial infection.22 Furthermore, as we have seen here, this impaired chemokine production affected recruitment of inflammatory monocytes to the site of infection.22 Inflammatory monocytes have been shown to differentiate into DCs23, 24 and macrophages,25 with the differentiation pathway of monocytes depending on the type of environmental and the nature of the infection.26 This prior study did not include an analysis of CD103+ DCs or a determination of whether the recruited monocytes differentiated into DCs or macrophages. Furthermore, this prior study also showed that epithelial cells infected with Citrobacter rodentium did not produce CCL2 and that CCL2 production is unique to stromal cells beneath the epithelium. However, this was determined using an epithelial cell line rather than primary epithelial cells. We were able to see epithelial-derived CCL2 by qPCR and ELISA, suggesting that differences may exist between primary cells and cell lines. The purity of CECs used in our study was >98%,27 making it highly unlikely that the CCL2 production detected was because of stromal cell contamination. Furthermore, previous work from our group has shown that epithelial-derived chemokine secretion is important in driving the recruitment of DCs to the large intestine,7 with the chemokines CCL5 and CCL20 particularly implicated. We observed lower levels of detectable chemokines from WT CECs in our model compared with our previously published data. In the previous study, only BALB/c and AKR mice were analyzed and not C57BL/6 mice. Thus, there may be differences in the different mouse strains, particularly when you consider that unlike BALB/c or AKR mice that have well-defined and polarized responses to T. muris, C57BL/6 mice have a mixed Th1/Th2 response. Furthermore, it is not clear that CCL5 is critical for DC recruitment in resistant BALB/c mice, as although blocking both CCL5 and CCL20 prevented DC recruitment, CCL5 was not tested alone. Other epithelial-derived chemokines may also be important in DC recruitment and it should be noted that our previous studies investigated CCL5, CCL20, CCL3, and CCL2,7 all of which were reduced in susceptible mice.
The defect in immune cell recruitment seen in Nod2−/− mice was specific to CD103+CD11c+MHChigh DCs and not basophils or macrophages. When looking at the MHCIIlow population, all cells were F4/80 negative. CD103+ MHCIIlow frequencies were low but were the same between Nod2−/− and C57BL/6 mice. In addition, we looked at CD11b expression by flow cytometry and immunohistochemistry and found it to be expressed on macrophages and DCs, and despite their being a small population of CD103+ CD11b+ cells in the large intestine, we did not observe differences in the frequency of these cells between Nod2−/− and C57BL/6 mice in early infection. This suggests Nod2 has an important role in CD103+ DC recruitment in the response to T. muris. Indeed, although macrophages have been shown to be recruited to the large intestine in T. muris infection, this is much later in infection (D21 after infection),28 suggesting they play different roles in orchestrating immunity to T. muris, and other mechanisms aside from Nod2 activation in the epithelium drive their recruitment or proliferation. DC migration to the site of infection is an important stage in the immune response,29 with many studies showing that DCs are necessary for priming adaptive immunity. For example, effective DC migration is needed to prevent susceptibility to infection with the protozoan parasite, Cryptosporidium parvum.30 Therefore, because of the observed impaired DC recruitment, we expected Nod2−/− mice to be susceptible to T. muris infection. Indeed, analysis of worm burdens at D21 after infection showed that Nod2−/− mice had delayed expulsion of T. muris. Furthermore, analysis of early T-cell responses at D7, D10, and D18 after infection demonstrated that whereas there was a clear response and expansion of T cells in the MLNs of WT mice at D18 after infection, this was significantly reduced in Nod2−/− mice. Strikingly, when we looked at the numbers of T cells positive for the gut-homing receptor CCR9, we observed a significantly lower number in Nod2−/− animals. This observation could explain why we see impaired parasite expulsion at D21 after infection. We suggest that impaired DC recruitment to the colon in the early stages of the immune response against T. muris then reduces priming of the early adaptive T-cell immune response in the MLNs. Thus, less T cells are primed and initiated to migrate to the gut that affects the initial stages of parasite expulsion. However, this deficit in DC and T-cell numbers is overcome as mice are ultimately able to expel infection, suggesting that reduced DC responses alter the dynamics of the immune response rather than the overall outcome to infection.
We show, via an in vitro chemotaxis assay, that DCs from both Nod2−/− and C57BL/6 mice are able to migrate effectively toward a chemotactic stimulus, suggesting that Nod2−/− DCs are functional, at least in their migratory capabilities. This, coupled with our data from the generation of bone marrow chimeras, allows us to propose that it is the Nod2-dependent epithelial responses that promote DC recruitment to the large-intestinal epithelium. This then could affect the downstream immune response such that there is a delayed expulsion of the parasite. Furthermore, although DC numbers did increase at D2 and D5 after infection in the Nod2−/− mice, the magnitude was not as great as that observed in WT mice. Thus, DC migration is both reduced and delayed in Nod2−/− mice. However, the small level of DC migration in the Nod2−/− mouse suggests that other mechanisms other than epithelial Nod2 signaling are involved in DC recruitment to the large intestine. For example, eosinophils have been shown to be important for DC recruitment to the lymph nodes that drain the lungs.31 As eosinophilia is also observed during T. muris infection,32 eosinophils may be involved in DC recruitment. Importantly, Nod2−/− mice were not devoid of DCs and DCs from Nod2−/− mice were shown in vitro to be capable of responding to T. muris antigen, thus suggesting that Nod2−/− DCs are still functioning effectively. In addition, as Nod2−/− mice are ultimately resistant to infection and able to expel all parasites by day 35 after infection, it may be that the few DCs recruited to the colon are sufficient to ultimately initiate an effective, although delayed, immunity. Indeed, coupled with delayed expulsion of the worm, Nod2−/− mice were able to mount an effective Th2 response and produced the cytokines IL-13 and IL-4 at equivalent levels to that seen in WT animals despite the impaired DC migration. This is not the first time that effective DC function has been shown to not be paramount to helminth resistance. Experiments restricting antigen presentation to DCs during T. muris infection did not impede host clearance of the pathogen or the ability of the host to mount Th2 responses.22 In these experiments, the role of antigen presentation and T-cell priming was attributed to basophils. We addressed whether, in the absence of an effective DC recruitment, there was a compensatory recruitment of basophils to the gut. However, our data demonstrated that basophils were detected at equivalent levels in the large intestine of both WT and Nod2−/− mice after infection, although in very low numbers. Previous data have shown a role for basophils in augmenting Th2 responses,33, 34 and therefore basophils may be acting to support the few CD103+ DCs observed in the Nod2−/− mice in promoting T-cell polarization.
The factors that drive the recognition of T. muris infection by Nod2 are still unknown, but there are several possibilities. PRRs have been shown to respond to damage-associated molecular patterns such as heat shock proteins, adenosine triphosphate, and heparin.8 T. muris physically invades the gastrointestinal epithelial through the aid of the secretion of pore-forming antigens.35, 36 During this process the epithelium is put under a lot of stress, potentially causing the release of damage-associated molecular patterns that may be detected by Nod2. Indeed, the NLR family member NLRP3 is known to detect damage-associated molecular patterns and cellular stress,37, 38 and previous work has shown synergism between NLRP3 and Nod2.39 Moreover, T. muris infection has been associated with increased apoptosis in susceptible mice,40 and E/S antigen from the porcine parasite Trichuris suis has been shown to have cytotoxic effects on intestinal epithelial cells.41 Combined, these data support our hypothesis that damage caused during infection may trigger Nod2 activation. T. muris also produces an E/S antigen that may contain components that have the potential to bind to Nod2; however, this is an unlikely hypothesis as our data from the CEC cultures failed to show any additional chemokine response to T. muris antigen. Another possibility is that Nod2 directly recognizes surface proteins on the helminth. Currently, we do not know what surface proteins are expressed on T. muris and if/how they interact with the epithelium or immune cells. It is not possible to maintain both T. muris larvae and viable CEC together in vitro, therefore these experiments cannot be performed, and thus this possibility cannot be ruled out yet. Finally, as the T. muris enters the epithelium, it may allow introduction of bacteria into the epithelial cells, either via opportunistic translocation of bacteria as pores are formed within the epithelial layer or T. muris could directly carry bacteria on its surface into the epithelium, thus triggering PRR engagement.
In conclusion, we have provided evidence that shows, for the first time, a role for a PRR in mediating the immune response to the pathogen T. muris. Activation of Nod2 mediates the production of epithelial-derived chemokines that then induces the recruitment of CD103+ DCs to the gastrointestinal epithelium. As DC recruitment to the epithelium has previously been shown to be important for host resistance to T. muris infection,7 activation of Nod2 is therefore potentially an important first step in the initiation of the immune response to infection.
METHODS
Mice. Male Nod2−/−C57BL/6 mice have been described previously42 and were bred in-house. Specific pathogen-free male C57BL/6 mice were purchased at 6–8 weeks of age from Harlan Olac (Bicester, UK). All mice were maintained by the Biological Services Unit (BSU), University of Manchester, UK, and kept in individually ventilated cages. Animals were treated and experiments performed according to the Home Office Animals (Scientific Procedures) Act (1986).
Parasites. Maintenance of the T. muris lifecycle and production of E/S antigen was carried out as described previously.43 Mice were infected with ∼175 embryonated eggs by oral gavage and killed at various time points after infection. Worm burdens were assessed as described previously.44
ELISA. T. muris–specific IgG1 and IgG2a antibodies were measured in sera samples collected at autopsy by ELISA using a previously described method.44 Chemokine production by colonic epithelial cells was measured by ELISA kit (R&D Systems, Abingdon, UK) for CCL2, CCL20, CCL5, and IL-33 according to the manufacturer’s instructions.
Histology. Cecal and colon snips were fixed in neutral buffered formalin for 24 h, processed, and embedded in paraffin wax. Then, 5 μm sections were dewaxed, rehydrated, and stained using a standard hematoxylin and eosin or periodic acid–Schiff stain. Slides stained with hematoxylin and eosin were measured for crypt hyperplasia, measured in 20 crypts per mouse using WCIF ImageJ software (http://imagej.nih.gov/ij). Goblet cells were counted in 20 crypts per mouse from periodic acid–Schiff–stained sections. All slides were measured and counted blind in a randomized order.
Immunofluorescence. Cecal and colonic snips were taken at autopsy and frozen in OCT embedding matrix (Thermo Fisher Scientific, Cheshire, UK). Then, 6 μm sections were fixed in 4% paraformaldehyde at 4 °C for 5 min. Sections were blocked using the tryamide blocking kit (PerkinElmer, Cambridge, UK) for 30 min. Endogenous biotins were blocked using the avidin/biotin blocking kit as per the manufacturer’s instructions (Vector Lab, Peterborough, UK). For four-color immunohistochemistry, slides were first stained with either purified anti-CD103 (Beckon Dickinson, Oxford, UK) and CD11b (Abcam, Cambridge, UK) or anti-F4/80 diluted in 0.1 M Tris-HCL (pH 7.5) (TNB). A secondary mouse-anti-rat IgG2a-Cy5 or anti-rabbit AF-488 (Invitrogen, Paisley, UK) was applied to slides. Slides were then incubated with the primary antibodies CD11c-biotin (eBioscience, Hatfield, UK) and cytokeratin-FITC (Sigma-Aldrich, Dorset, UK). Samples were incubated with streptavidin–horseradish peroxidase for 30 min. After washing, samples were incubated with Tyramide Cy3 detection antibody for 5 min. Slides were washed and mounted with vector shield containing 4’,6-diamidino-2-phenylindole (Vector Lab). Slides were imaged and CD11c+, or CD11c+CD103+ or CD11c+F4/80+ counted per field of view in a blind randomized order. Three to four fields/view were counted per section.
MLN cell culture and cytokine analysis. Single-cell suspensions were prepared from MLNs taken at autopsy and added at 5 × 106 cells per well in 1 ml cultures to 48-well plates and stimulated with T. muris E/S at 50 μg ml−1. Cells were incubated at 37 °C, 5% CO2, 95% humidity for 48 h, after which time the supernatants were harvested and stored at −20 °C. For cytokine analysis, levels of IL-4, IL-10, IL-6, IL-9, IL-13, interferon-γ, tumor necrosis factor-α, and IL-12p70 were determined using a custom cytometric bead array according to the manufacturer’s instructions (CBA, Becton Dickinson (BD), Oxford, UK) and analyzed using BD FacsAria cytometer and FCAP Array software. For flow cytometry analysis, single-cell suspensions were prepared. Total cell numbers were counted and the cells were resuspended at 5 × 106 cells per ml. Cells were washed and Fc receptors were blocked using anti-CD16/32 (2 μg ml−1; eBioscience). Cells were washed with anti-CD4, anti-CD8, CD44, CD62L, and anti-CCR9 (eBioscience) and acquired by flow cytometry on the BD LSRII. Data were analyzed using FlowJo flow cytometry software (Tree Star Ashland, OR).
Generation of bone marrow chimeras. Recipient mice were irradiated with two 5-Gy doses 4 h apart and injected intravenously with bone marrow harvested from donor mice at 10 million cells per 250 μl sterile phosphate-buffered saline. Bone marrow was allowed to reconstitute for 6 weeks before mice were infected with T. muris. Verification of successful reconstitution of bone marrow chimeras was determined by analysis of microbe-associated molecular pattern and MDP responsiveness of isolated bone marrow cells. Bone marrow was harvested and cultured as previously described.45 On D6 of the culture, the cells were harvested from the plates and semi-adherent cells removed. The cells were spun at 400 g and resuspended at a concentration of 1 × 106 cells per ml in DC medium (RPMI-1640 supplemented with 10% LPS-free fetal bovine serum (Gibco, Paisley, UK), 1% penicillin/streptomycin, and 50 mM β-mercaptoethanol (Sigma-Aldrich)). The cells were stimulated overnight with LPS (100 ng ml−1) or MDP (5 μg ml−1). After 24 h, the cells were harvested and prepared for flow cytometry. FcRs were blocked by incubating cells in anti-CD16/32 (2 μg ml−1) for 15 min. Cells were washed and stained with anti-CD45 PeCy7 (1 μg ml−1, Becton Dickinson), anti-CD11c Alexa700 (1 μg ml−1), anti-MHC-II FITC (2.5 μg ml−1), and anti-CD86 PE (1 μg ml−1; all from eBioscience) and acquired using a LSRII flow cytometer (Becton Dickinson Biosciences, Oxfordshire, UK). Data were analyzed using FlowJo flow cytometry analysis software (Tree Star).
Large intestine cell isolation and flow cytometry. Cecum and colon were harvested at autopsy and digested in RPMI containing 5% L-glutamine, 5% penicillin streptomycin, 10% fetal bovine serum (Sigma-Aldrich), collagenase (1 mg ml−1), and dispase (0.5 mg ml−1, both from Gibco) for 2 h at 37 °C. Cells were then forced through a 70 μm cell strainer, centrifuged at 405 g for 5 min, and resuspended in 10 ml 80% Percoll (GE Healthcare, Buckinghamshire, UK) solution that was then overlaid on a 40% Percoll solution. Cells were centrifuged for 25 min at 1,000 g and the cells at the gradient interface harvested. Fc receptors were blocked using anti-CD16/32 (2 μg ml−1; eBioscience). Cells were washed and stained with anti-CD45 PeCy7 (1 μg ml−1, Becton Dickinson), anti-CD103 PE (1 μg ml−1), anti-CD11c Alexa700 (2.5 μg ml−1), anti-MHC-II FITC (2.5 μg ml−1), and anti-F4/80 APC (1 μg ml−1) (all from eBioscience) and acquired by flow cytometry on the BD LSRII. Data were analyzed using FlowJo flow cytometry software (Tree Star).
Colonic and cecal cell isolation, complementary DNA conversion, and qPCR. Cecum and colon were harvested at autopsy, digested in RPMI containing 5% L-glutamine, 5% penicillin streptomycin, 10% fetal bovine serum, and dispase (1 mg ml−1 Gibco) for 90 min at 37 °C. Cells were then forced through a 70 μm cell strainer (Becton Dickinson) and spun at 400 g for 5 min and resuspended in 1 ml TRIsure (Bioline, London, UK). Epithelial purity was confirmed by flow cytometry using antibodies against CD326 (Ep-CAM, Cambridge Bioscience, Cambridge, UK) and CD45-PE (Becton Dickinson). All epithelial preparations were >98% pure. Total RNA was isolated from cells by homogenizing in TRIsure, phases separated using chloroform (Sigma-Aldrich) and RNA precipitated in isopropanol (Sigma-Aldrich). RNA concentration was analyzed on a nanodrop-1000 spectrophotometer (Labtech International, East Sussex, UK) and resuspended at a concentration of 1 μg μl−1 using Bioscript–M-MLV kit (Bioscript, London, UK) for complementary DNA conversion. Quantitative PCR was performed using the SYBR green I core kit (Eurogentec, Southampton, UK) and an Opticon quantitative PCR thermal cycler (Bio-Rad, Hemel Hempstead, UK). Each sample was serially diluted, and expression ratios normalized to the mean of two reference primers (Gapdh and Ywhaz). Primer sequences are given in Table 1.
CEC culture. Monolayer cultures of primary CECs were cultured as described previously.27 After 24 h, the cells were incubated with T. muris E/S antigen 50 μg ml−1, T. muris eggs (10 eggs per well), or medium for 24 h. The supernatant was then harvested and analyzed by ELISA.
Bone marrow DC culture. Bone marrow was harvested and cultured as previously described.45 On D6 of culture, cells were harvested from the plates and semi-adherent cells removed. The cells were spun at 400 g and resuspended at a concentration of 1 × 106 cells per ml in DC medium (RPMI-1640 supplemented with 10% LPS-free fetal bovine serum (Gibco), 1% penicillin/streptomycin, and 50 mM β-mercaptoethanol (Sigma Aldrich)). The cells were stimulated overnight with LPS (100 ng ml−1) or T. muris antigen (5 μg ml−1). After 24 h, the cells were harvested and prepared for flow cytometry. FcRs were blocked by incubating cells in anti-CD16/32 (2 μg ml−1) for 15 min. Cells were washed and stained with anti-CD45 PeCy7 (1 μg ml−1, Becton Dickinson), anti-CD11c Alexa700 (1 μg ml−1), anti-MHC-II FITC (2.5 μg ml−1), and anti-CD86 PE (1 μg ml−1; all from eBioscience), and acquired using a LSRII flow cytometer (Becton Dickinson Biosciences). Data were analyzed using FlowJo flow cytometry analysis software (Tree Star).
Chemokine migration assay. Colonic lamina propria cells from WT or Nod2−/− mice were labeled with Vybrant fluorescent dye (Molecular Probes, Leiden, The Netherlands) and added to the upper well of transwell plates (Fisher Scientific, Loughborough, UK) at 1–8 × 105 per well and chemokines were added to the bottom well (CCL2 (10 ng ml−1) and CCL2 (1 ng ml−1), both from R&D Systems). As a control, cells were also plated in the absence of chemokine. After incubating for 1 h at 37 °C, cells in the bottom well were stained with CD11c antibodies and the total number of dye-labeled cells counted using an Olympus BX51 (Southend-on-Sea, UK) upright microscope, captured using a Coolsnap ES camera (Photometrics, Tucson, AZ) through MetaVue Software (Molecular Devices, Berkshire, UK).
Statistics. Statistical analyses were performed using Student’s t-test, two-way analysis of variance, or Kruskal–Wallis test with Dunn’s multiple comparison test. The P-values of <0.05 were considered significant. All statistical analyses were carried out using GraphPad Prism for windows, version 3.02 (La Jolla, CA).
References
Crompton, D.W.T. How much human helminthiasis is there in the world? J. Parasitol. 85, 397–403 (1999).
Cliffe, L.J. & Grencis, R.K. The Trichuris muris system: a paradigm of resistance and susceptibility to intestinal nematode infection. Adv. Parasitol. 57, 255–307 (2004).
Mempel, T.R., Henrickson, S.E. & Von Andrian, U.H. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427, 154–159 (2004).
Hammad, H & Lambrecht, B.N. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat. Rev. Immunol. 8, 193–204 (2008).
Rimoldi, M et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat. Immunol. 6, 507–514 (2005).
deSchoolmeester, M.L., Manku, H & Else, K.J. The innate immune responses of colonic epithelial cells to Trichuris muris are similar in mouse strains that develop a type 1 or type 2 adaptive immune response. Infect. Immun. 74, 6280–6286 (2006).
Cruickshank, S.M. et al. Rapid dendritic cell mobilization to the large intestinal epithelium is associated with resistance to Trichuris muris infection. J. Immunol. 182, 3055–3062 (2009).
Lotze, M.T. et al. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol. Rev. 220, 60–81 (2007).
Chen, G et al. NOD-like receptors: role in innate immunity and inflammatory disease. Annu. Rev. Pathol. 4, 365–398 (2009).
Gutierrez, O et al. Induction of Nod2 in myelomonocytic and intestinal epithelial cells via nuclear factor-κB activation. J. Biol. Chem. 277, 41701–41705 (2002).
Inohara, N et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. J. Biol. Chem. 278, 5509–5512 (2003).
Caetano, B.C. et al. Intrinsic expression of Nod2 in CD4+T lymphocytes is not necessary for the development of cell-mediated immunity and host resistance to Toxoplasma gondii. Eur. J. Immunol. 41, 3627–3631 (2011).
Ekman, A.-K. & Cardell, L.O. The expression and function of Nod-like receptors in neutrophils. Immunology 130, 55–63 (2010).
Cruickshank., S, Wakenshaw, L, Cardone., J, Howdle., P, Murray., P & Carding., S Evidence for the involvement of NOD2 in regulating colonic epithelial cell growth and survival. World J. Gastroenterol. 14, 5834–5841 (2008).
Sabbah, A et al. Activation of innate immune antiviral responses by Nod2. Nat. Immunol. 10, 1073–1080 (2009).
Shaw, M.H. et al. T cell-intrinsic role of Nod2 in promoting type 1 immunity to Toxoplasma gondii. Nat. Immunol. 10, 1267–1274 (2009).
Kobayashi, K.S. et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307, 731–734 (2005).
Petnicki-Ocwieja, T et al. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc. Natl. Acad. Sci. USA 106, 15813–15818 (2009).
Rehman, A et al. Nod2 is essential for temporal development of intestinal microbial communities. Gut 60, 1354–1362 (2011).
Perrigoue, J.G. et al. MHC class II-dependent basophil-CD4+ T cell interactions promote TH2 cytokine-dependent immunity. Nat. Immunol. 10, 697–705 (2009).
Else, K.J., Hultner, L & Grencis, R.K. Cellular immune responses to the murine nematode parasite Trichuris muris. II. Differential induction of TH-cell subsets in resistant versus susceptible mice. Immunology 75, 232–237 (1992).
Kim, Y.-G. et al. The Nod2 sensor promotes intestinal pathogen eradication via the chemokine CCL2-dependent recruitment of inflammatory monocytes. Immunity 34, 769–780 (2011).
Narni-Mancinelli, E et al. Memory CD8+ T cells mediate antibacterial immunity via CCL3 activation of TNF/ROI+ phagocytes. J. Exp. Med. 204, 2075–2087 (2007).
Rivollier, A et al. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J. Exp. Med. 209, 139–155 (2012).
Arnold, L et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 204, 1057–1069 (2007).
Gordon, S & Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 (2005).
Baumgart, D.C. et al. Mechanisms of intestinal epithelial cell injury and colitis in interleukin 2 (IL-2)-deficient mice. Cell. Immunol. 187, 52–66 (1998).
Little, M.C. et al. The characterization of intraepithelial lymphocytes, lamina propria leukocytes, and isolated lymphoid follicles in the large intestine of mice infected with the intestinal nematode parasite Trichuris muris. J. Immunol. 175, 6713–6722 (2005).
Jung, S et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 17, 211–220 (2002).
Auray, G et al. Involvement of intestinal epithelial cells in dendritic cell recruitment during C. parvum infection. Microbes Infect. 9, 574–582 (2007).
Jacobsen, E.A. et al. Eosinophils regulate dendritic cells and Th2 pulmonary immune responses following allergen provocation. J. Immunol. 187, 6059–6068 (2011).
Svensson, M et al. Accumulation of eosinophils in intestine-draining mesenteric lymph nodes occurs after Trichuris muris infection. Parasite Immunol. 33, 1–11 (2011).
Kim, S et al. Cutting edge: basophils are transiently recruited into the draining lymph nodes during helminth infection via IL-3, but infection-induced Th2 immunity can develop without basophil lymph node recruitment or IL-3. J. Immunol. 184, 1143–1147 (2010).
Torrero, M.N. et al. Basophils amplify type 2 immune responses, but do not serve a protective role, during chronic infection of mice with the filarial nematode Litomosoides sigmodontis. J. Immunol. 185, 7426–7434 (2010).
Drake, L et al. The major secreted product of the whipworm, Trichuris, is a pore-forming protein. Proc. Biol. Sci. 257, 255–261 (1994).
Drake, L.J. et al. Molecular and functional characterization of a recombinant protein of Trichuris trichiura. Proc. Biol. Sci. 265, 1559–1565 (1998).
Tschopp, J & Schroder, K NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 10, 210–215 (2010).
Leemans, J.C., Cassel, S.L. & Sutterwala, F.S. Sensing damage by the NLRP3 inflammasome. Immunol. Rev. 243, 152–162 (2011).
Conforti-Andreoni, C et al. Synergism of NOD2 and NLRP3 activators promotes a unique transcriptional profile in murine dendritic cells. J. Leukoc. Biol. 88, 1207–1216 (2010).
Cliffe, L.J. et al. An increase in epithelial cell apoptosis is associated with chronic intestinal nematode infection. Infect. Immun. 75, 1556–1564 (2007).
Abner, S.R. et al. Response of intestinal epithelial cells to Trichuris suis excretory/secretory products and the influence of campylobacter jejuni invasion under in vitro conditions. J. Parasitol. 88, 738–745 (2002).
Pauleau, A.-L. & Murray, P.J. Role of Nod2 in the response of macrophages to Toll-like receptor agonists. Mol. Cell. Biol. 23, 7531–7539 (2003).
Wakelin, D Acquired immunity to Trichuris muris in the albino laboratory mouse. Parasitology 57, 515–524 (1967).
Else, K et al. MHC-restricted antibody responses to Trichuris muris excretory/secretory (E/S) antigen. Parasite Immunol. 12, 509–527 (1990).
Lutz, M.B. et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223, 77–92 (1999).
Acknowledgements
This study was supported by the Biotechnology and Biological Sciences Research Council, UK (to S.C.) and Epistem. R.G. was supported by the Wellcome Trust grant number 083620/Z/07/Z. A special thanks to Dr Kaye Williams and Epistem for their help with the generation of bone marrow chimeras.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Supplementary information
Rights and permissions
About this article
Cite this article
Bowcutt, R., Bramhall, M., Logunova, L. et al. A role for the pattern recognition receptor Nod2 in promoting recruitment of CD103+ dendritic cells to the colon in response to Trichuris muris infection. Mucosal Immunol 7, 1094–1105 (2014). https://doi.org/10.1038/mi.2013.125
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2013.125
This article is cited by
-
The role of β2 integrin in dendritic cell migration during infection
BMC Immunology (2021)
-
Epithelial extracellular ATP: an initiator of immunity to parasitic infections
Immunology & Cell Biology (2017)
-
P2X7 receptor‐dependent tuning of gut epithelial responses to infection
Immunology & Cell Biology (2017)
-
NOD2 dependent neutrophil recruitment is required for early protective immune responses against infectious Litomosoides sigmodontis L3 larvae
Scientific Reports (2016)