Abstract
Neonatal Fc receptors for immunoglobulin (Ig)G (FcRn) assume a central role in regulating host IgG levels and IgG transport across polarized epithelial barriers. We have attempted to elucidate the contribution of FcRn in controlling Helicobacter infection in the stomach. C57BL/6J wild-type or FcRn−/− mice were infected with Helicobacter heilmannii, and gastric lesions, bacterial load and the levels of antigen-specific IgG in serum and gastric juice were analyzed. The elevated levels of anti-H. heimannii IgG in gastric juice were observed exclusively in wild-type mice but not in FcRn−/− mice. In contrast, an increase in lymphoid follicles and bacterial loads along with deeper gastric epithelium invasion were noted in FcRn−/− mice. C57BL/6J wild-type or FcRn−/− mice were also infected with Helicobacter pylori SS1, and the results of the bacterial load in stomachs of these mice and the anti-H. pylori IgG levels in serum and gastric juice were similar to those from H. heilmannii infection. Our data suggest that FcRn can be functionally expressed in the stomach, which is involved in transcytosis of IgG, and prevent colonization by H. heilmannii and the associated pathological consequences of infection.
Similar content being viewed by others
Introduction
Secretory immunoglobulins (Igs), such as IgA, IgM, and IgG, that are present in mucosal surfaces, potentially provide a first line of defense against microorganisms.1, 2, 3 Secretory IgA (sIgA) is well-known to be transported across epithelial cells into the lumen through an active unidirectional process accompanying the polymeric Ig receptor.4 In addition to sIgA, mucosal secretions of the human gastrointestinal, respiratory, and genital tracts contain significant quantity of IgG. In a previous study, it was reported that nasal secretions contain 300 μg ml−1 of IgG,4 and ∼800 μg ml−1 of IgG was detected in the human rectum.5 Similar to sIgA, which has been well documented as a factor actively participating in the defense against some pathogens,3, 4, 5, 6 the mucosally associated IgG has also been recently suggested to contribute to host defense.1, 2 So far, the transport of IgA to mucosa and its involvement in mucosal host defense have been well understood, but the role of gastric luminal IgG in defending against enteric bacteria and the relationship between IgG and bacterial colonization remains to be established. Previously, it was revealed that IgG is transported across intact epithelial barriers through the placenta in humans and the neonatal intestine in rodents for the passive transfer of immunity from mother to the fetus or neonatal infant.7, 8 The receptor responsible for mediating this transport system is the neonatal Fc receptor for IgG (FcRn), which is structurally related to the major histocompatibility complex class I molecules, and is a heterodimer composed of a glycosylated heavy (α) chain associated noncovalently with β2-microglobulin.9 Fc-hinge fragments of IgG at the CH2–CH3 domain interface have a central role in its binding to FcRn.10 FcRn mainly have four cellular functions: the bidirectional transport of IgG across epithelial cells, the protection of IgG from catabolism, the protection of albumin from catabolism and antigen presentation by dendritic cells.10 Human FcRn is the vehicle through which IgG is transported across the intestinal epithelium, and recycle the IgG–antigen complex back across the intestinal epithelial barrier into the lamina propria (LP) for processing by dendritic cells and presentation to CD4+ T cells.2 The transport of IgG through FcRn may regulate immune responses to luminal pathogens. In a previous study, it was revealed that the transport of IgG and the antigen–IgG complex by FcRn has an important role in the immune defense against Citrobacter rodentium infection.11 This previous report indicates that the transport of the anti-bacterial IgG antibodies via FcRn leads to the direct protection against bacterial invasion from the epithelium into LP indirectly by affecting antigen presentation to antigen-specific T cells followed by the activation and proliferation of antigen-specific CD4+ T cells. The activation and proliferation aid in the killing of invading bacteria, and also lead to the differentiation of immature B cells into plasma cells for the production of bacterial antigen-specific IgGs.
Helicobacter heilmannii (H. heilmannii), which is a Gram-negative rod bacterium that belongs to the Helicobacter family including Helicobacter pylori (H. pylori), is characterized by a relatively large size (5–9 μm) and a corkscrew appearance. H. heilmannii colonizes human gastric mucosa at a relatively low rate (0.5–6%),12 and leads to gastritis,13 malignant lymphoma,14 and mucosa-associated lymphoid tissue (MALT) lymphoma.15 Interestingly, a clinical study revealed that primary gastric MALT lymphoma occurred more frequently in H. heilmannii-infected patients (1.47%) than in H. pylori-infected patients (0.66%).16 The infection with H. heilmannii was also observed in the gastric mucosa of various mammals, including cats, dogs, pigs, and nonhuman primates,17, 18 strongly being suspected to be a zoonotic agent. Microorganisms are superficially located within the mucous layer without adhesion to epithelial cells. In our previous study, H. heilmannii was detected in the relatively deep part of the foveolae,19 whereas H. pylori preferentially localized in a layer containing mucin derived from surface mucus cells.20 Interestingly, in one case, intracytoplasmic H. heilmannii organisms were observed in parietal cells with cell damage.21 Thus, the infection site of H. heilmannii is different from that of H. pylori, and accordingly H. heilmannii must be an interesting investigation object. So far, it remains to be determined whether and how both FcRn and bacterial antigen-specific IgGs regulate the bacterial infection in the gastric mucosal tissue. Therefore, in this report, the roles of FcRn in the transport of bacterial antigen-specific IgGs in the gastric tissue were examined using FcRn knockout mice (FcRn−/− mice). Then, a pathophysiological role of FcRn-mediated IgG secretion into the gastric lumen in H. heilmannii infection was investigated.
Results
Expression of FcRn in gastric epithelial cells
Recently, it has been reported that FcRn constitutively expresses in epithelial cells of human intestine, lung, and kidney,22, 23 but not the stomach. Therefore, the expression of FcRn in the gastric mucosa of mice was investigated. As shown in Figure 1a, the expressions of FcRn mRNA and protein were observed in the gastric mucosa by RT-PCR (Figure 1a) and immunoblot, respectively (Figure 1b). An immunohistochemical staining revealed that the ratio of FcRn expression in the gastric epithelial surface was relatively high, and its location within endocytic organelles in epithelial cells was also observed (Figure 1c). Next, the epithelial cells were isolated from the mouse stomach, and then the expression of FcRn in gastric epithelial cells was evaluated. As a result, the FcRn expression in gastric epithelial cells obtained from FcRn+/+ mice and FcRn+/− mice was observed, and the expression level in FcRn+/+ mice was higher than in FcRn+/− mice (Figure 1d,e), although its expression level in gastric epithelial cells was lower than in whole stomach. In FcRn−/− mice, FcRn expression was not detected. It was reported that the histological analysis of liver, lung, kidney, and lymphoid organs of adult FcRn−/− mice is normal,24 and actually adult FcRn−/− and the wild-type mice were indistinguishable based on body weight and health condition.
Expression of neonatal Fc receptors for IgG (FcRn) in the gastric mucosa. (a) mRNA from the stomach of FcRn+/+, FcRn+/−, or FcRn−/− mice (each n=5) was obtained, and then subjected to RT-PCR using FcRn- or β-actin-specific primers. FcRn: 164 bp; and β-actin: 110 bp. Typical images are shown. (b) Proteins were obtained from the stomach of FcRn+/+, FcRn+/−, or FcRn−/− mice (each n=5), and subjected to immunoblotting using an anti-FcRn antibody. Molecular weight of FcRn: 46 kDa. Typical images are shown. (c) Immunohistochemical staining was performed by confocal laser scanning microscopy (each n=5). Green: FcRn and blue: F-actin. Original magnification × 630. Typical images are shown. (d) mRNA from gastric epithelial cells isolated from FcRn+/+, FcRn+/−, or FcRn−/− mice (each n=3) was obtained, and then subjected to RT-PCR using FcRn or β-actin-specific primers. FcRn: 164 bp and β-actin: 110 bp. Typical images are shown. (e) mRNA from the stomach of FcRn+/+, FcRn+/−, or FcRn−/− mice (each n=3) and mRNA from gastric epithelial cells isolated from FcRn+/+, FcRn+/−, or FcRn−/− mice (each n=3) were subjected to real-time quantitative PCR. The FcRn level was normalized to that of β-actin as an internal standard. Data are presented as mean±s.e. for each group (n=3).
FcRn-mediated transport of IgG across the epithelial barrier
It was examined whether FcRn in gastric epithelial cells can transport IgG into mucosal secretions, because FcRn was demonstrated to be involved in the secretion of IgG from tissue spaces into the lumen through epithelial cells in mice expressing human FcRn under the control of an epithelial cell specific promoter.2 To determine whether the intravenous (IV)-injected IgG can be transported into the lumen across the epithelial barrier by FcRn-dependent transcytosis, FcRn+/+ and FcRn−/− mice were used. In this study, rabbit IgG or phosphate-buffered saline (PBS) as a vehicle control was IV injected into FcRn+/+ and FcRn−/− mice, because mouse FcRn can bind rabbit IgG as well as mouse IgG.25 The levels of rabbit IgG in the gastric juice were examined 6 h after the IV injection. We observed that the level of rabbit IgG was significantly higher in the gastric juice of FcRn+/+ mice injected with rabbit IgG compared with PBS-injected FcRn+/+ mice, and there were no differences in the levels of rabbit IgG in the gastric juice between the rabbit IgG- and PBS-injected FcRn−/− mice (Figure 2). These results indicate that the majority of IgG transport into gastric secretion is dependant upon FcRn expression. In the absence of FcRn, low levels of IgG reach the lumen presumably by exudation.
The levels of immunoglobulin (Ig)G secreted into the gastric lumen as determined by expression of neonatal Fc receptors for IgG (FcRn) in the stomach. Six hours after intravenous (IV) injection of rabbit IgG or phosphate-buffered saline (PBS) as a vehicle control into FcRn+/+ and FcRn−/− mice (each n=5), the levels of rabbit IgG in the gastric secretion of the mice were measured by enzyme-linked immunosorbent assay. Data are represented as mean±s.e. (n=5). *P<0.05 according to Student's t-test.
FcRn-deficient mice are susceptible to H. heilmannii infection
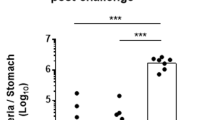
In previous studies, it has been shown that IgG may have a critical role in prevention against bacteria related to infectious diseases.26, 27, 28 H. heilmannii is also a bacterial pathogen, which causes gastritis, peptic ulcer, acute gastric mucosal lesion, gastric carcinoma, and MALT lymphoma.29 To test a pathophysiological role of FcRn-mediated IgG secretion into the gastric lumen in the H. heilmannii infection, FcRn+/+ and FcRn−/− mice were orally inoculated with H. heilmannii. The presence of H. heilmannii, but not H. pylori, infection was confirmed using H. heilmannii- and H. pylori-specific 16S rRNA primers before conducting any experiment (Figure 3a). Four weeks after infection, the level of anti-H. heilmannii IgG in the serum and gastric juice was measured using enzyme-linked immunosorbent assay. The higher level of anti-H. heilmannii IgG was detected in the gastric secretion of FcRn+/+ mice compared with FcRn−/− mice (Figure 3b), although there were no differences in the level of anti-H. heilmannii IgG in the serum of both H. heilmannii-infected FcRn+/+ and FcRn−/− mice (Figure 3c). In addition, in the paraffin-fixed gastric sections of H. heilmannii-infected FcRn+/+ mice, the higher level of IgG was observed in the epithelial and subepithelial tissue in comparison with that observed in the non-infected FcRn+/+ mice and the infected FcRn−/− mice, although IgG was detected in a part of gastric lumen in the infected FcRn−/− mice (Figure 4).
Levels of anti-Helicobacter heilmannii immunoglobulin (Ig)G in the serum and gastric juice of FcRn−/− and FcRn+/+ mice 4 weeks after H. heilmannii infection. FcRn+/+ and FcRn−/− mice (each n=5) were infected with H. heilmannii for 4 weeks as described in Methods. (a) PCR using DNA samples extracted from homogenates of gastric mucosa of FcRn+/+ and FcRn−/− mice was performed with the H. heilmannii type1 16S r-RNA primers or the H. pylori 16S r-RNA primers. β-Actin was used as a control. Typical images are shown. (b, c) The level of anti-H heilmannii IgG in the serum (b) and gastric juice (c) of FcRn−/− and FcRn+/+ mice was measured by enzyme-linked immunosorbent assay. Data are represented as mean±s.e. (n=5). *P<0.05 according to Student's t-test. FcRn, neonatal Fc receptors for IgG.
Immunohistochemical staining of anti-Helicobacter heilmannii immunoglobulin (Ig)G in the stomach of FcRn−/− and FcRn+/+ mice 4 weeks after H. heilmannii infection. FcRn+/+ and FcRn−/− mice (each n=5) were infected with H. heilmannii as described in Methods. After 4 weeks, the stomach was resected and opened at the outer curvature. The stomach was sliced longitudinally from the esophagus to the duodenum, and then embedded in paraffin wax. The sections (100 μm) were cut, and selected serial sections of H. heilmannii-infected and non-infected FcRn+/+ and FcRn−/− mice were stained with goat anti-mouse IgG antibody in a blind manner. Original magnification × 200. Typical images are shown. FcRn, neonatal Fc receptors for IgG.
FcRn-deficient mice exhibit deep bacterial invasion of H. heilmannii into the gastric mucosa
Next, the relationship between the anti-H. heilmannii IgG antibody and bacterial localization within the gastric mucosa was examined. Four weeks after H. heilmannii infection, the stomach of the FcRn+/+ and FcRn−/− mice was obtained, after careful fixation of the gastric mucosa by Carnoy's fixation, confocal microscopic analysis of H. heilmannii in the gastric sections was performed using a cross-reacting polyclonal rabbit anti-H. pylori antibody. Increased amounts of H. heilmannii within deep pits in the gastric epithelium of the FcRn−/− mice were observed. In contrast, H. heilmannii infection in FcRn+/+ mice was limited to the surface of the gastric epithelium (Figure 5).
Immunohistochemical staining of Helicobacter heilmannii in the stomach of FcRn−/− and FcRn+/+ mice 4 weeks after H. heilmannii infection. Immunohistological examination of the gastric mucosa from FcRn+/+ and FcRn−/− mice (each n=5) 4 weeks after H. heilmannii infection was performed by confocal laser scanning microscopy. The stomach was treated as described in the Figure legend of Figure 4, and the selected serial sections were stained with polyclonal rabbit anti-H. pylori antibody in a blind manner. Green: H. heilmannii, red: F-actin, and blue: nucleus. Original magnification × 630. Typical images are shown. FcRn, neonatal Fc receptors for IgG.
FcRn-deficient mice are more susceptible to the formation of lymphoid follicles with H. heilmannii infection
Inability to secrete IgGs into the gastric lumen in the absence of FcRn expression in gastric epithelium may limit the ability of FcRn−/− mice to properly manage H. heilmannii infection. Therefore, FcRn+/+ and FcRn−/− mice were infected with H. heilmannii for 6 months, and the degree of epithelial injury in H. heilmannii-infected FcRn+/+ and FcRn−/− mice was evaluated. By macroscopic examination, more severe mucosal projection consistent with lymphoid follicles were observed in H. heilmannii-infected FcRn−/− mice in comparison with FcRn+/+ mice (Figure 6a). This was confirmed by showing that the number (Figure 6b), but not the size, (Figure 6c) of lymphoid follicles in the stomach of the FcRn−/− mice was higher than that of the FcRn+/+ mice. Consistent with these macroscopic changes, H. heilmannii-infected FcRn−/− mice exhibited increased infiltration of mononuclear cells and neutrophils into the gastric tissue, together with increased epithelial injury in comparison with the FcRn+/+ mice (Figure 6d). Hematoxylin and eosin staining in Figure 6d showed that gastric epithelial cells in FcRn−/− mice were morphologically identical to those in FcRn+/+ mice. In addition, expression levels of epithelial-specific molecules, such as epithelial-specific Epcam and Cytokeratin Type 1, in FcRn−/− mice were the same as those in FcRn+/+ mice (data not shown), suggesting that FcRn−/− mice have the similar distribution of epithelial cell lineages as FcRn+/+ mice. These results indicate that an absence of FcRn expression in the gastric epithelium and consequently IgG in the lumen leads to the greater susceptibility to H. heilmannii infection and the formation of gastric lymphoid follicles.
Pathological characterization of the gastric mucosal surfaces of FcRn−/− and FcRn+/+ mice chronically infected with Helicobacter heilmannii. FcRn+/+ and FcRn−/− mice (each n=9) were infected with H. heilmannii as described in Methods. (a) After 6 months, the stomach mucosa was stained with an indigo carmine solution. Typical images are shown. (b, c) After 6 months, the total number (b) and the major axis size (c) of lymphoid follicles per the stomach of the mice was determined macroscopically. Data are represented as mean±s.e. (n=9), and *P<0.05 according to Student's t-test. (d) Histological examination of the gastric mucosa in the corpus region of FcRn+/+ and FcRn−/− mice 6 months after H. heilmannii infection was performed by hematoxylin and eosin staining. Original magnification × 200. Typical images are shown. FcRn, neonatal Fc receptors for IgG.
Anti-H. heilmannii IgG decreases the bacterial load via FcRn in gastric epithelial cells
The above experiments revealed that the defense against an epithelial-associated pathogen in the stomach is dependent on the expression of FcRn. As FcRn−/− mice lacked high levels of the secreted IgG, to test whether IgG specific to H. heilmannii is required for the regulation and elimination of H. heilmannii, H. heilmannii was orally inoculated into FcRn+/+ and FcRn−/− mice that received the IV injection of either a polyclonal cross-reactive rabbit anti-H. pylori antibody or a control rabbit antibody, and the amount of H. heilmannii in the FcRn+/+ and FcRn−/− mice was evaluated on the basis of the level of H. heilmannii 16S r-RNA present in the gastric mucosa. In the control rabbit antibody administration, there was more H. heilmannii mRNA in the FcRn−/− mice than in the FcRn+/+ mice (Figure 7). In addition, the pre-administration of an anti-H. pylori antibody reduced the bacterial levels in the gastric lumen in the FcRn+/+ mice, but it was not the case in FcRn−/− mice (Figure 7). These results indicate that H. heilmannii-specific IgG could prevent deep H. heilmannii colonization in the LP, and the clearance mechanism might be via capturing of H. heimannii by dead epithelial cells that are sloughed off from the infected mucosa.
Evaluation of Helicobacter heilmannii in the stomach of FcRn−/− and FcRn+/+ mice at 1 week after passive transfer of antibody. FcRn+/+ and FcRn−/− mice (each n=5) were infected with H. heilmannii as described in Methods. After 1 week, mRNA was obtained from the stomach of H. heilmannii -infected FcRn+/+ and FcRn−/− mice, and the levels of H. heilmannii type1 16S r-RNA were determined by real-time quantitative PCR. The 16S r-RNA level was normalized to those of β-actin as an internal standard. FcRn+/+ and FcRn−/− mice (each n=5) were also subjected to intravenous (IV) injection of polyclonal rabbit anti-H. pylori antibody or control rabbit antibody in the 5th and 6th days after H. heilmannii infection. Data are presented as mean±s.e. for each group (n=5). *P<0.05 according to Student's t-test. FcRn, neonatal Fc receptors for IgG.
Infection of FcRn+/+ and FcRn−/− mice with H. pylori strain SS1
Finally, to investigate the role of IgG in well-known helicobacter bacteria H. pylori, FcRn+/+ and FcRn−/− mice were infected with H. pylori strain SS1. Eight weeks after infection, the level of anti-H. pylori IgG in the serum and gastric juice of FcRn−/− and FcRn+/+ mice was measured by enzyme-linked immunosorbent assay (Figure 8), and the levels of H. pylori 16S r-RNA in the stomach were also determined by real-time quantitative PCR (Figure 9). As a result, the level of anti-H. pylori IgG in the gastric juice of H. pylori-infected FcRn−/− mice was lower than in H. pylori-infected FcRn+/+ mice, but the serum anti-H. pylori IgG level was not different between the H. pylori-infected FcRn+/+ mice and FcRn−/− mice (P=0.830; Figure 8). In addition, the number of H. pylori in the stomach of FcRn−/− mice was larger than FcRn+/+ mice (Figure 9). These results were similar to those found from H. heilmannii infection. The histological examination also revealed that the degree of infiltration of inflammatory cells in the H. pylori SS1-infected FcRn−/− mice was relatively more severe than the FcRn+/+ mice, although tissue damage was not observed (Figure 10a). In an immunofluorescence staining with an anti-H. pylori antibody, the presence of H. pylori was confirmed in the stomach of FcRn−/− and FcRn+/+ mice infected with H. pylori SS1, but the prominent difference in its location between FcRn−/− and FcRn+/+ mice was not observed (Figure 10b), being different from the results of H. heilmannii.
The level of anti-Helicobacter pylori IgG in the serum and gastric juice of FcRn−/− and FcRn+/+ mice 8 weeks after H. pylori infection. FcRn+/+ and FcRn−/− mice (each n=4) were infected with H. pylori SS1 for 8 weeks as described in Methods. The levels of anti-H. pylori IgG in the serum (a) and gastric juice (b) of FcRn−/− and FcRn+/+ mice were measured by enzyme-linked immunosorbent assay. Data are represented as mean±s.e. (n=4). *P<0.05 according to the Student's t-test. FcRn, neonatal Fc receptors for IgG.
Evaluation of the amount of Helicobacter pylori SS1 colonization in the stomach of FcRn−/− and FcRn+/+ mice 8 week after H. pylori infection. FcRn+/+ and FcRn−/− mice (each n=5) were infected with H. pylori SS1 as described in Methods. After 8 weeks, mRNA of the stomach of H. pylori-infected FcRn+/+ and FcRn−/− mice were obtained, and then the levels of H. pylori 16S r-RNA were determined by real-time quantitative PCR. The levels were normalized to those of β-actin as an internal standard. Data are represented as the mean±s.e. for each group (n=5). *P<0.05 according to Student's t-test. FcRn, neonatal Fc receptors for IgG.
Immunohistochemical staining of Helicobacter pylori SS1 in the stomach of FcRn−/− and FcRn+/+ mice 8 weeks after H. pylori SS1 infection. (a) Histological examination of the gastric mucosa in the corpus region of FcRn+/+ and FcRn−/− mice (each n=5) 8 weeks after H. pylori SS1 infection was performed by hematoxylin and eosin staining in a blind manner. Original magnification × 200. Typical images are shown. (b) Immunohistological examination of the gastric mucosa from FcRn+/+ and FcRn−/− mice (each n=5) 8 weeks after H. pylori SS1 infection was performed by confocal laser scanning microscopy in a blind manner. Green: H. pylori; Red: F-actin; and Blue: nucleus. Original magnification × 630. Typical images are shown.
Discussion
The recent recognition that FcRn expresses in mucosal epithelial cells of adult humans and that FcRn mediates an important physiological process such as bidirectional transport of IgG30, 31 have led to the notion that the in vivo functions of FcRn are broader than the simple acquisition of passive immunity. They are further supported by the recent demonstration that the human FcRn expressed as a transgene in mice under the control of its endogenous human promoter can mediate the transport of a model antigen OVA from the lumen.2 These results raise the possibility that important physiological functions of FcRn are associated with the digestive system against exposures to a wide spectrum of enteric commensal and pathogenic bacteria. In this study, we attempted to define the role of gastric epithelial cell-associated FcRn. As a result, we not only detected the expression of FcRn in gastric epithelial cells but also could confirm the transport of IgG via FcRn into gastric secretion. Moreover, it was shown that FcRn is required for the transport of bacterial antigen-specific IgGs and furthermore H. heilmannii-specific IgG could prevent deep H. heilmannii colonization in the LP. These results indicate that FcRn in the gastric tissue is required for defense against this important pathogen.
The distinctive histological features of H. heilmannii-associated gastritis include the followings: an absence of epithelial damage,32 lymphocytic exudation into gastric foveolae,33 a mild inflammatory activity,34 infrequent development of MALT,35, 36 and rarely intestinal metaplasia.14 In contrast to gastritis, several studies revealed that the formation of lymphoid follicles or B-cell MALT lymphoma is the characteristic lesion for H. heilmannii infection in mice,35, 36 although its severity is varied. In the present study, the degree of formation of lymphoid follicles was significantly higher in H. heilmannii-infected FcRn−/− mice in comparison with the infected C57BL/6J wild-type mice (Figure 6a). Given that gastric luminal secretion of IgG was abrogated in FcRn−/− mice, the absence of FcRn expression resulted in increased numbers of H. heilmannii in the stomach of FcRn−/− mice (Figure 7). An administration of bacterial-specific IgG, i.e., H. heilmannii cross-reacting anti-H. pylori antibody,37 led the elimination of this epithelial cell-associated pathogen in wild-type mice compared with the control rabbit IgG administration but not FcRn−/− mice (Figure 7). These results indicate as follows: in FcRn+/+ mice, the IV-administered anti-H. pylori antibody was transferred to gastric mucosa via FcRn, resulting in the decreased H. heilmannii level. The same phenomena was not observed in FcRn−/− mice, because the IV-administered anti-H. pylori antibody was not transported to gastric mucosa in FcRn−/− mice. H. heilmannii infection led to the production of anti-H. heilmannii antibody, i.e., antigen-specific antibody, and in FcRn+/+ mice, the anti-H. heilmannii antibody was transferred to gastric mucosa through FcRn, but in FcRn−/− mice this transport was not caused because of the absence of FcRn, resulting in higher level of H. heilmannii in FcRn−/− mice than in FcRn+/+ mice. It is likely that FcRn-mediated secretion of IgG eliminates this epithelial cell-associated pathogen and its consequence MALT. In this regard, Park and Hong36 examined the gastric distribution of Ig-secreting cells during Helicobacter suis (H. suis) infection, and these investigations revealed a large number of IgA-positive cells in the LP, especially of the upper mucosa, and the corpus submucosa in H. suis-infected mice, but not in the control mice. IgG1-, IgG2b-, and IgM-positive cells were rarely detected in the gastric mucosa of H. suis-infected and the control mice.38 However, these findings differed from the results by Peterson et al.,35 who exhibited that the number of IgG- and IgM-positive cells was increased in gastric mucosa of BALB/c mice infected with H. heilmannii. In outbred Swiss mice infected with Helicobacter felis, increased numbers of gastric IgA- and IgG-positive cells were also observed, although the number of IgM-positive cells was not increased.39 It can also be assumed that the types of Ig-secreting cells during Helicobacter infection are dependent upon the bacterial strain and possibly genetic background of the host as another variable in determining whether IgG is available for FcRn-dependent transport. In our previous report, it was demonstrated that B cells in the gastric follicles of H. heilmannii-infected mice were CD45R-positive.19 Possibly, there are few IgG-producing B cells in the stomach, including the parts of the formed lymphoid follicles, because there are the lower level of IgG (Figure 4) and the larger number of lymphoid follicles (Figure 6) in the stomach of H. heilmannii-infected FcRn−/− mice. Further studies on the participated types of Ig-secreting cells during H. heilmannii infection are therefore required.
Mechanistically, our studies predict that the gastric luminal IgG delivered from the LP by the FcRn-mediated transport across the epithelia directly contributes to the suppression of H. heilmannii infection. Thus, gastric FcRn may have a role in host defense by transporting IgG into a place that inhibits the adhesion and/or invasion of the bacterium. The absence of the anti-H. heilmannii IgG transport into gastric mucosa in FcRn−/− mice leads to the deeper gastric epithelial invasion of H. heilmannii (Figure 5) together with a larger number of H. heilmannii (Figure 7) in the gastric epithelium of the infected FcRn−/− mice. On the basis of current results, together with previous studies,2, 15, 21, 35 we would suggest the following model for H. heilmannii infection. Following H. heilmannii infection, the host senses the presence of H. heilmannii either through heterologous antibody binding to antigen by cross-reacting IgG antibodies and apical to basal transportation of these immune complexes or through dendritic cell acquisition of bacteria directly from the lumen, consequently leads to an adaptive immune response in association with the production of IgG anti-H. heilmannii antibodies. Anti-H. heilmannii IgG is subsequently transported into the gastric epithelium or lumen via FcRn thus directing IgG to a site required for its anti-bacterial effect or action. In the absence of FcRn, anti-H. heilmannii IgG is not delivered into the gastric tissue, leading to deeper gastric epithelial invasion of H. heilmannii and furthermore to the aggravation of H. heilmannii-associated diseases such as MALT. In our experiments, it was confirmed that the mRNA expression level of FcRn tended to be increased up to about 1.8 times (n=3; P=0.079 by Student’s t-test) under 4 weeks-infection with H. heilmannii, and this increase may be also involved in FcRn-dependent protection against H. heilmannii infection.
The interesting findings were derived from comparison of H. heilmannii and H. pylori infections in FcRn−/− mice. The bacterial levels in their infected FcRn−/− mice was higher than the infected FcRn+/+ mice (Figures 7,9), and the gastric juice levels in their infected FcRn−/− mice was lower than their infected FcRn+/+ mice (Figures 3,8), suggesting the possibility of an association with Helicobacter bacteria and FcRn. In a previous study, Garhart et al.40 and Sutton et al.41 demonstrated that antibodies do not seem to be effective for H. pylori-evoked gastritis, suggesting the possibility that cellular immunity is important for protection against H. pylori infection. However, there might be also a relationship between the Helicobacter bacterial level and the FcRn-dependent transport of IgG, because the number of H. pylori was larger in FcRn−/− mice (Figure 9). Possibly, the H. pylori level is little dependent in H. pylori-evoked diseases, because immunization of H. pylori lysates to B-cell-deficient μMT mice could decrease the bacterial level, although the immunization could not affect H. pylori-evoked gastritis.40 Interestingly, in contrast to H. pylori, H. heilmannii could invade into deep pits in the gastric epithelium and enhance the formation of gastric lymphoid follicles in absence of FcRn. These results indicate that H. heilmannii but not H. pylori can enhance the pathogenesis of diseases through its deep invasion, and these phenomena might be involved in the big differences between H. heilmannii and H. pylori in the pathogenesis of infectious diseases.
In this study, we provide an insight into the role of FcRn on the transport of secreted bacterial-specific IgG into gastric tissues. FcRn in the gastric epithelium is the primary means by which IgG reaches the gastric lumen as seen by the absence of luminal IgG in the context of FcRn deficiency. Moreover, in relation to a gastric epithelial infection, this FcRn-mediated IgG transport likely has a significant role in defending against epithelial-associated pathogens as shown here. In conclusion, H. heilmannii-specific IgG transported via FcRn into the gastric tissue has an important role in defending against H. heilmannii infection and its complications. Consequently understanding the functions of FcRn in the stomach will lead to the development of the therapeutic strategies against a gastric epithelial cell-associated pathogen, such as H. heilmannii.
Methods
Mice. All animal experiments were performed according to the “Guidelines for Animal Experimentation” at the Kobe University (Permission No. P-090907). FcRn−/− mice were established previously,24 and kindly provided by Dr Derry Roopenian (Jackson Laboratory, Bar Harbor, Maine).C57BL/6J mice; i.e., FcRn+/+ mice, were purchased from CLEA Japan (Shizuoka, Japan), and were bred under standard laboratory conditions.
H. heilmannii infection. FcRn+/+ and FcRn−/− mice (6 to 8 weeks old) were infected with H. heilmannii, which was originally obtained from Cynomolgus monkey and was genetically identified as H. heilmannii as described previously.15 Confirmation of H. heilmannii infection was performed with PCR using DNA samples extracted from mucosal homogenates and primers for H. heilmannii type 1 16S r-RNA; i.e., 5′-TTGGGAGGCTTTGTCTTTCCA-3′ and 5′-GATTAGCTCTGCCTCGCGGCT-3′. A control experiment was performed using primers for H. pylori 16S r-RNA; i.e., 5′-TGCGAAGTGGAGCCAATCTT-3′ and 5′-GGAACGTATTCACCGCAACA-3′. To confirm H. heilmannii infection, immunohistological examination was also performed using a cross-reacting anti-H. pylori antibody as reported previously.37
H. pylori SS1 infection. FcRn+/+ and FcRn−/− mice (6 to 8 weeks old) were infected with H. pylori SS1 (2 × 108 CFU per one injection) twice for the second successive day. Confirmation of H. pylori SS1 infection was performed with PCR using DNA samples extracted from mucosal homogenates and primers for H. pylori 16S rRNA gene primers; i.e., 5′-TGCGAAGTGGAGCCAATCTT-3′ and 5′-GGAACGTATTCACCGCAACA-3′. To confirm H. pylori SS1 infection, immunohistological examination was also performed using a cross-reacting anti-H. pylori antibody as reported previously.40
Preparation of isolated gastric epithelial cells. Gastric epithelial cells were isolated and confirmed according to a previous report.42 Briefly, the mouse stomach were collected into Ca2+- and Mg2+-free Hanks balanced salt solution with 5% fetal calf serum, penicillin and streptomycin, and then transferred into Hanks balanced salt solution containing 1 mM dithiothreitol and 1 mM EDTA. After incubation for 1 h at 37 °C, the resulting cell suspensions were washed with PBS, and then collected as gastric epithelial cells.
Determination of rabbit IgG, H. heilmannii - and H. pylori SS1-specific IgG in the serum and gastric juice by enzyme-linked immunosorbent assay. Rabbit anti-OVA antibody (Rockland, Gilbertsville, PA) was used as rabbit IgG. Six hours after IV injection of 1 mg rabbit anti-OVA antibody into FcRn+/+ and FcRn−/− mice, the gastric juice was collected. For detection of rabbit anti-OVA antibody in the gastric juice, 96-well plates were coated overnight at 4 °C with 100 μl of a bicarbonate solution (pH 9.6) containing 100 μg ml−1 OVA (Sigma, St Louis, MO). After washing with PBS containing 0.05% Tween 20 (PBST), the plates were blocked by the addition of 1.5% bovine serum albumin (BSA) in PBS for 1 h at 37 °C. The plates were then washed twice with PBST before the gastric juice diluted at 1:400 in PBST containing 0.2% BSA was added. The plates were then incubated for 2 h at 37 °C. Next, the plates were washed twice with PBST, followed by addition of 100 μl of donkey anti-rabbit IgG antibody conjugated with horseradish peroxidase (Thermo Scientific, Rockford, IL) diluted at 1:5000 in PBST containing 0.2% BSA. After incubation for 2 h at 37 °C, the plates were twice washed with PBST, and then the bound antibody was detected by addition of O-phenylenediamine substrate, followed by measurement of absorbance at 490 nm. To detect H. heilmannii- and H. pylori SS1-specific IgG in the serum and gastric juice of FcRn+/+ and FcRn−/− mice 4 weeks after H. heilmannii and 8 weeks after H. pylori SS1 infection, hyaluronidase in PBS (0.3 mg μl−1) was added to the stomach followed by dissolution of the mucus layer. The gastric juice was collected and, then centrifuged at 16,000 × g for 5 min at 4 °C, and the resultant supernatant was collected. Therefore, the gastric juice obtained includes molecules that remain below the mucus in the zone of cytoprotection. The serum was also separated from the blood by centrifugation at 15,000 × g for 10 min at 4 °C. Ninety six-well plates were coated overnight at 4 °C with 100 μl of a bicarbonate solution (pH 9.6) containing 100 μg ml−1 H. pylori lysate, and blocked by the addition of 1.5% BSA in PBS for 1 h at 37 °C. The serum and gastric juice, which was diluted at 1:200 and 1:15, respectively, was added to the plates, followed by addition of 100 μl of hRP-conjugated goat anti-mouse IgG antibody (Bio-Rad Laboratories, Hercules, CA) diluted at 1:5,000 in PBST containing 0.2% BSA. The bound antibody was detected by addition of O-phenylenediamine substrate, and measurement of absorbance at 490 nm was carried out.
Immunohistochemistry. Four weeks after H. heilmannii infection, the gastric mucous layer was fixed by Carnoy's fixation after the stomach of mice was resected and opened at the outer curvature, and the sections were prepared as described previously.43 Briefly, the stomach was sliced longitudinally from the esophagus to the duodenum, and then embedded in paraffin wax. The sections (100 μm) were cut and, the selected serial sections (3–5 sections for one sample obtained from one mouse) were used in a blind manner. Next, an endogenous peroxidase activity was blocked by addition of 0.3% H2O2 in methanol for 30 min before the sections were blocked with 5% BSA in PBS, and the sections were incubated with primary antibodies; i.e., biotin-conjugated goat anti-rabbit IgG antibody (Pierce, Rockford, IL) diluted at 1:500 for detection of rabbit IgG and biotin-conjugated goat anti-mouse IgG antibody (Pierce) diluted at 1:500 for detection of mouse IgG for 1 h. After washing with PBS, the sections were incubated with a streptavidin solution (DAKO, Osaka, Japan) for 1 h. The chromogenic reaction was developed with 3-diaminobenzidine (Sigma Chemical, Poole, UK) containing 0.02% H2O2 and the sections were mounted in synthetic medium. The negative control was obtained by omitting primary antibodies.
Immunofluorescence staining. Paraffin-embedded 4 weeks H. heilmannii and 8 weeks, after H. pylori SS1-infected, gastric tissues were fixed with a Carnoy’s solution to preserve the surface mucous gel layer after the stomach of mice was resected and opened at the outer curvature. The stomach was sliced longitudinally from the esophagus to the duodenum, and then embedded in paraffin wax. The paraffin-embedded gastric tissues were de-paraffinized in xylene and were immersed in absolute ethanol, before 100 μm thick tissue sections were cut using a tissue microslicer (Microslicer DTK-1000; Dosaka EM, Kyoto, Japan), and the selected serial sections (3–5 sections for 1 sample obtained from one mouse) were used in a blind manner. The sections were rinsed in 0.1 M phosphate buffer (pH 7.4) containing 0.1 M NaCl three times, and then were immersed for 30 min in PBS containing BSA at a concentration of 1 mg ml−1. The sections were incubated with rabbit anti-mouse FcRn (SantaCruz Biotechnology, SantaCruz, CA) diluted at 1:100 overnight at 4 °C, followed by reaction with goat-anti rabbit IgG antibody labeled with Alexa 488 (Invitrogen, Carlsbad, CA). The fluorescence was visualized by a confocal laser scanning microscope (Zeiss LSM510; Carl Zeiss, Oberkochen, Germany).
Histological examination. Six months after H. heilmannii infection, the stomach was resected and opened at the greater curvature before being stained with an indigo carmine solution. Subsequently, the number and major axis size of clearly identified gastric lymphoid follicles in each stomach were evaluated. Half of the stomach was embedded in paraffin wax, and the paraffin-embedded tissues were sliced and stained with hematoxylin and eosin in a blind manner. Eight weeks after H. pylori SS1 infection, half of the stomach was embedded in paraffin wax, and the paraffin-embedded tissues were sliced and stained with hematoxylin and eosin in a blind manner.
Quantitative real-time PCR. H. heilmannii- and H. pylori-infected mucosal and submucosal layers of the stomach, and the isolated gastric epithelial cells were homogenized with 1 ml of TRIZOL reagent (Invitrogen), and RNA was extracted from the homogenates according to the manufacturer's instructions. RNA was subjected to the reverse transcription reaction using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) according to the manufacturer's protocols, and quantitative real-time PCR was performed using Power SYBR Green PCR Master Mix. The following primers were used: β-actin: 5′-AAGGCCAACCGTGAAAAGAT-3′ and 5′-GTGGTACGACCAGAGGCATAC-3′; H. heilmannii type 1 16S rRNA: 5′-AGACAAAGCCTCCCAACAAC-3′ and 5′-ATCACTGACGCTGATTGCAC-3′; H. pylori 16S rRNA: 5′-TGCGAAGTGGAGCCAATCTT-3′ and 5′-GGAACGTATTCACCGCAACA-3′; FcRn: 5′-CAGCCTCTCACTGTGGACCTAGA-3′ and 5′-TCGCCGCTGAGAGAAAGC-3′; epithelial-specific epcam: 5′-GAGCGCGTGAGGACCTACTGGA-3′ and 5′-TGTGAACGCCTCTTGAAGCGCA-3′; cytokeratin Type 1: 5′-CAGAGAGCAGTCAGCCGCCAT-3′ and 5′-GTGCTGGGCCTGCAGGTCAAT-3′. To allow a relative comparison of RNA expression levels, the data from real-time PCR were normalized to the amount of β-actin cDNA as an endogenous control.
Statistical analysis. All results are expressed as means±s.e. Statistical significance was analyzed using the Student's t-test, and a level of probability of 0.05 was used as the criterion of significance.
References
Robert-Guroff, M. IgG surfaces as an important component in mucosal protection. Nat. Med. 6, 129–130 (2000).
Yoshida, M. et al. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity 20, 769–783 (2004).
Woof, J.M. & Mestecky, J. Mucosal immunoglobulins. Immunol. Rev. 206, 64–82 (2005).
Hanson, L.A. & Brandzaeg, P. The mucosal defense system. In Immunologic Disorders In Infants And Children. ( Stiehm E.R., eds W.B. Saunders: Pennsylvania, 137–164 (2011 ).
Kozlowski, P.A., Cu-Uvin, S., Neutra, M.R. & Flanigan, T.P. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect. Immun. 65, 1387–1394 (1997).
Brandtzaeg, P. & Johansen, F.E. Mucosal B cells: phenotypic characteristics, transcriptional regulation, and homing properties. Immunol. Rev 206, 32–63 (2005).
Story, C.M., Mikulska, J E. & Simister, N.E. A major histocompatibility complex class I-like Fc receptor cloned from human placenta: possible role in transfer of immunoglobulin G from mother to fetus. J Exp Med. 180, 2377–2381 (1994).
Simister, N.E. & Rees, A.R. Isolation and characterization of an Fc receptor from neonatal rat small intestine. Eur J Immunol. 15, 733–738 (1985).
Simister, N.E. & Mostov, K.E. An Fc receptor structurally related to MHC class I antigens. Nature 337, 184–187 (1989).
Qiao, S.W. et al. Dependence of antibody-mediated presentation of antigen on FcRn. Proc Natl Acad Sci USA 105, 9337–9342 (2008).
Yoshida, M. et al. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J. Clin. Invest. 116, 2142–2151 (2006).
O'Rourke, J.L., Lee, A. & Kellow, J.E. Infections of the gastrointestinal tract. In: Infection M. J. Blaser, P. D. Smith, J. I. Ravdin, H. B. Greenberg and R. L. Guerrant, Eds. ( Raven Press: New York, 523–533, 2002 ).
Mazzucchelli, L., Wilder-Smith, C.H., Ruchti, C., Meyer, W.D. & Merki, H.S. Gastrospirillum hominis in asymptomatic, healthy individuals. Dig Dis Sci 38, 2087–2089 (1993).
Stolte, M., Kroher, G., Meining, A., Morgner, A., Bayerdörffer, E. & Bethke, B. A comparison of Helicobacter pylori and H. heilmannii gastritis. A matched control study involving 404 patients. Scand J Gastroenterol. 32, 28–33 (1997).
Nakamura, M. et al. Candidatus Helicobacter heilmannii from a cynomolgus monkey induces gastric mucosa-associated lymphoid tissue lymphomas in C57BL/6 mice. Infect Immun. 75, 1214–1222 (2007).
Morgner, A. et al. Helicobacter heilmannii-associated primary gastric low-grade MALT lymphoma: complete remission after curing the infection. Gastroenterology 118, 821–828 (2000).
De Groote, D. et al. ‘Candidatus Helicobacter suis’, a gastric helicobacter from pigs, and its phylogenetic relatedness to other gastrospirilla. Int J Syst Bacteriol. 49, 1769–1777 (1999).
O'Rourke, J.L., Dixon, M.F., Jack, A., Enno, A. & Lee, A. Gastric B-cell mucosa-associated lymphoid tissue (MALT) lymphoma in an animal model of Helicobacter heilmannii infection. J Pathol. 203, 896–903 (2004).
Nobutani, K. et al. Helicobacter heilmannii can induce gastric lymphoid follicles in mice via a Peyer's Patch independent pathway. FEMS. Immunol. Med. Microbiol. 60, 156–164 (2010).
Hidaka, E. et al. Helicobacter pylori and two ultrastructurally distinct layers of gastric mucous cell mucins in the surface mucous gel layer. Gut 49, 474–480 (2001).
Joo, M. et al. Helicobacter heilmannii-associated gastritis: clinicopathologic findings and comparison with Helicobacter pylori-associated gastritis. J. Korean Med. Sci. 22, 63–69 (2007).
Dickinson, B.L. et al. Bidirectional FcRn dependent IgG transport in a polarized human intestinal epithelial cell line. J. Clin. Invest. 104, 903–911 (1999).
Israel, E.J. et al. Expression of the neonatal Fc receptor, FcRn, on human intestinal epithelial cells.. Immunology 92, 69–74 (1997).
Roopenian, D.C. et al. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J. Immunol. 170, 3528–3533 (2003).
Ober, R.J., Radu, C.G., Ghetie, V. & Ward, E.S. Differences in promiscuity for antibody-FcRn interactions across species: implications for therapeutic antibodies. Int. Immunol. 13, 1551–1559 (2001).
Bry, L. & Brenner, M.B. Critical role of T cell-dependent serum antibody, but not the gut-associated lymphoid tissue, for surviving acute mucosal infection with Citrobacter rodentium, an attaching and effacing pathogen. J. Immunol. 172, 433–441 (2004).
Maaser, C. et al. Clearance of Citrobacter rodentium requires B cells but not secretory immunoglobulin A (IgA) or IgM antibodies. Infect.Immun. 72, 3315–3324 (2004).
Simmons, C.P. et al. Central role for B lymphocytes and CD4+ T cells in immunity to infection by the attaching and effacing pathogen Citrobacter rodentium. Infect. Immun. 71, 5077–5086 (2003).
Nishikawa, K. et al. Increased apoptosis and angiogenesis in gastric low-grade mucosa-associated lymphoid tissue-type lymphoma by Helicobacter heilmannii infection inC57/BL6mice. FEMS Immunol Med Microbiol 50, 268–272 (2007).
Claypool, S.M. et al. Bidirectional transepithelial IgG transport by a strongly polarized basolateral membrane Fc gamma-receptor. Mol. Biol. Cell. 15, 1746–1759 (2004).
Claypool, S.M., Dickinson, B.L., Yoshida, M., Lencer, W.I. & Blumberg, R.S. Functional reconstitution of human FcRn in Madin-Darby canine kidney cells requires coexpressed human beta 2-microglobulin. J. Biol. Chem. 277, 28038–28050 (2002).
Holck, S. et al. The histopathology of human gastric mucosa inhabited by Helicobacter heilmannii-like (Gastrospirillum hominis) organisms, including the first culturable case. APMIS. 105, 746–56 (1997).
Ierardi, E. et al. Helicobacter heilmannii gastritis: a histological and immunohistochemical trait. J Clin Pathol. 54, 774–777 (2001).
Jhala, D., Jhala, N., Lechago, J. & Haber, M. Helicobacter heilmannii gastritis: association with acid peptic diseases and comparison with Helicobacter pylori gastritis. Mod Pathol. 12, 534–538 (1999).
Peterson, R.A., Danon, S.J. & Eaton, K.A. Comparison of gastritis and gastric epithelial proliferation in Helicobacter heilmannii-infected nude and BALB/c mice. Vet Pathol. 38, 173–183 (2001).
Park, J.H. & Hong, J.J. Experimental infection of mice with tightly coiled spiral bacteria (‘‘Candidatus Helicobacter suis’’) originating from the pig stomach. J Comp Pathol. 129, 154–160 (2003).
Okiyama, Y., Matsuzawa, K., Hidaka, E., Sano, K., Akamatsu, T. & Ota, H. Helicobacter heilmannii infection: clinical, endoscopic and histopathological features in Japanese patients. Pathol Int. 55, 398–404 (2005).
Park, J.H., Seok, S.H., Baek, M.W., Lee, H.Y. & Kim, D.J. Gastric lesions and immune responses caused by long-term infection with Helicobacter heilmannii in C57BL/6 Mice. J. Comp. Path. 139, 208–217 (2008).
Ferero, R.L., Ave, P., Radcliff, F.J., Labigne, A. & Huerre, M.R. Outbred mice with long-term Helicobacter felis infection develop both gastric lymphoid tissue and glandular hyperplastic lesions. J Pathol. 191, 333–340 (2000).
Garhart, C.A., Nedrud, J.G., Heinzel, F.P., Sigmund, N.E. & Czinn, S.J. Vaccine-induced protection against Helicobacter pylori in mice lacking both antibodies and interleukin-4. Infect. Immun. 71, 3628–3633 (2003).
Sutton, P., Wilson, J., Kosaka, T., Wolowczuk, I. & Lee, A. Therapeutic immunization against Helicobacter pylori infection in the absence of antibodies. Immunol. Cell Biol. 78, 28–30 (2000).
Wang, J. et al. Helicobacter pylori modulates lymphoepithelial cell interactions leading to epithelial cell damage through Fas/Fas ligand interactions. Infect Immun. 68, 4303–4311 (2000).
Ota, H. & Katsuyama, T. Alternating laminated array of two types of mucin in the human gastric surface mucous layer. Histochem J. 24, 86–92 (1992).
Acknowledgements
This work was supported by grants for the Global COE Program, Global Center of Excellence for Education and Research on Signal Transduction Medicine in the Coming Generation (T.A and M.Y), Scientific Research in Priority Areas ‘Genome’ (T.A and M.Y), and Grant-in-Aid for Scientific Research on Innovative Areas (T.A) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and partly for the COE research support program from Hyogo prefecture (T.A.). R.S.B. was supported by NIH R01 DK053056.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Rights and permissions
About this article
Cite this article
Ben Suleiman, Y., Yoshida, M., Nishiumi, S. et al. Neonatal Fc receptor for IgG (FcRn) expressed in the gastric epithelium regulates bacterial infection in mice. Mucosal Immunol 5, 87–98 (2012). https://doi.org/10.1038/mi.2011.53
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2011.53
This article is cited by
-
The therapeutic age of the neonatal Fc receptor
Nature Reviews Immunology (2023)
-
Distribution of immunoglobulin G antibody secretory cells in small intestine of Bactrian camels (Camelus bactrianus)
BMC Veterinary Research (2015)
-
Anti-CXCL13 antibody can inhibit the formation of gastric lymphoid follicles induced by Helicobacter infection
Mucosal Immunology (2014)
-
The Immunologic Functions of the Neonatal Fc Receptor for IgG
Journal of Clinical Immunology (2013)