Abstract
Single-phased, high-color-rendering index (CRI) white-light phosphors are emerging as potential phosphor-converted white-light-emitting diodes (WLEDs) and as an alternative to blends of tricolor phosphors. However, it is a challenge to create a high CRI white light from a single-doped activator. Here, we present a high CRI (Ra = 91) white-light phosphor, Sr5(PO4)3-x(BO3)xCl:Eu2+, composed of Sr5(PO4)3Cl as the beginning member and Sr5(BO3)3Cl as the end member. This work utilized the solid-solution method, and tunable Eu2+ emission was achieved. Color-tunable Eu2+ emissions in response to structural variation were observed in Sr5(PO4)3-x(BO3)xCl solid solutions. This was further confirmed using X-ray Rietveld refinement, electron paramagnetic resonance spectroscopy, and in the photoluminescence spectra. The color-tunable emissions included the white light that originated from the combination of the blue emission of Sr5(PO4)3Cl:Eu2+ and an induced Eu2+ yellow emission at approximately 550 nm in the solid solution. Importantly, the white-light phosphors showed a greater R9 = 90.2 under excitation at 365 nm. This result has rarely been reported in the literature and is greater than that of (R9 = 14.3) commercial Y3Al5O12:Ce3+-based WLEDs. These findings demonstrate the great potential of Sr5(PO4)3-x(BO3)xCl:0.04Eu2+ as a white-light phosphor for near-UV phosphor-converted WLEDs. These results also provide a shortcut for developing a high CRI white-light phosphor from a single Eu2+-doped compound.
Similar content being viewed by others
Introduction
White-light-emitting diodes (WLED) fabricated with near ultraviolet LED (NUVLED) and tricolor phosphors are a potential substitute for current lighting sources1,2,3. However, these types of WLED devices are rather complicated, and the color balance is difficult to control4. Recently, single-phased, white-light phosphors have attracted more attention because they avoid most of these problems4. A conventional strategy using single-phased white-light phosphors is to co-dope ion pairs based on the energy transfer principle, e.g., Eu2+→+Mn2+, Eu2+→Tb3+/Mn2+, Ce3+→Mn2+, and Ce3+→Tb3+/Mn2+ pairs5,6,7,8,9,10,11,12,13,14. In these systems, the common feature is that Mn2+ is the red-emitting activator. However, the weak Mn2+ d-d transitions usually lead to additional excitation quenching paths to reduce the quantum yield (QY) and thermal stability15,16,17,18. A single-doping activator-activated white-light phosphor that suppresses the energy loss related to energy transfer is highly desired for phosphor-converted WLEDs. However, it is difficult to achieve white light from a compound with a single-doped activator that must also cover the entire visible lighting range from 400 nm to 700 nm.
Solid-solution phosphors are an efficient tool with which to explore white-light phosphors activated by a single-doped activator because this phosphor has a variable composition. This variable composition can simultaneously alter multiple parameters of the host lattice and thereby induce multiple crystal field environments imposed on the emission centers. By utilizing this strategy, some novel phosphors and new luminescence phenomena have been identified, such as tunable emission color, efficient luminous output, and improved thermal/chemistry stability19,20,21,22,23,24,25. For solid-solution phosphors, the end members usually must have high crystal chemical flexibility, or the end members are nearly isotypic despite crystallizing in structures with distinct space groups. Sr5(PO4)3Cl is an important member of the apatite-type halophosphates M5(XO4)3Cl compounds (M = Ca, Sr, Ba, X = P, V, Si) that has been widely investigated for nearly five decades8,26,27,28,29. The compound crystallizes in a hexagonal structure with a P63/m space group, can incorporate various foreign ions, and can provide abundant crystal field environments26,27,28,29. Sr5(BO3)3Cl crystallizes in an orthorhombic structure with a C2221 space group that has stoichiometrically mimicked apatite-type compounds and has been studied for almost two decades30. Sr5(BO3)3Cl also presents a rich crystal chemistry due to the wide range of possible cationic substitution30. Therefore, a novel oxychloride solid solution with a solid-solution containing the two traditional Sr5(PO4)3Cl and Sr5(BO4)3Cl compounds is possible based on their crystal chemistry similarity. Sr5(PO4)3Cl:Eu2+ is a highly efficient blue-emitting phosphor for WLEDs8, whereas Sr5(BO4)3Cl:Eu2+ phosphor presents a broadband red emission near 615 nm31. Therefore, a color-tunable emission that includes white light is expected from this oxychloride solid-solution compound.
In this study, the Eu2+-activated Sr5(PO4)3-x(BO3)xCl solid-solution phosphors comprised of Sr5(PO4)Cl as the oxychloride beginning member and Sr5(BO4)3Cl as the end member were synthesized via a solid-state reaction. Their structures were investigated using powder X-ray diffraction (XRD), XRD Rietveld refinement analysis, and electron paramagnetic resonance (EPR). The optical properties of the phosphors are discussed in detail using crystal structure analysis, photoluminescence (PL) excitation (PLE), transition decays, and fluorescence microscopy images. As expected, a tunable emission including white light with a high color rendering index (CRI; Ra = 91, R9 = 90.2) was achieved from the single-Eu2+-doped Sr5(PO4)3-x(BO3)xCl solid solution at an excitation of 365 nm. To the best of our knowledge, these are the greatest indices for white-light phosphors reported, especially for those obtained by single-Eu2+ doping of an oxychloride compound. This study provides a shortcut for developing a high CRI white-light phosphor from a single-Eu2+-doped compound. In addition, this work promotes the development of single-phased white-light phosphors.
Materials and Methods
Materials synthesis
The Sr5(PO4)3-x(BO3)xCl powders were made using solid-state reaction routes. The starting materials were SrCl2⋅6H2O (99.95%), H2BO3 (99.99%), SrHPO4 (99.95%), SrCO3 (99.95%), and Eu2O3 (99.99%). The powder mixtures were quantitatively mixed using an agate mortar and pestle, and were subsequently sintered for 3 h at 1350 °C in a CO reducing atmosphere. The products were reground and fired again for 1 h at 1350 °C in a muffle furnace and were then ground again for further characterization.
Characterization methods
The structures of the samples were identified using a Rigaku D/max-2500 X-ray diffractometer (Rigaku Co., Japan) with Cu Kα radiation (λ = 0.15406 nm). The detector covers an angular range 10° < 2θ < 105° with a counting time of 5 s per step. The Rietveld refinement used a general structure analysis system (GSAS) program32. The peak shapes were handled using a pseudo-Voigt function with Finger-Cox-Jephcoat asymmetry to correct for axial divergence at low angles. The crystal constants of the samples were calculated using X-ray Rietveld refinement. The room temperature PL spectra were measured with a homemade PL measurement system consisting of a 365-nm NUVLED array as the excitation source and an Ocean Optics USB 4000 plug-and-play spectrometer. The PLE spectra were analyzed using a Horiba FL-3 fluorescence spectrophotometer equipped with a 450 W Xe lamp. Fluorescence microscope PL images (TE-2000 Instruments; Nikon, Japan) of the powder particles were recorded under dark-field mode using a 365-nm UV lamp excitation. Fluorescence decay time curves were measured using an Edinburgh FLS920 spectrophotometer with an nF900 flash lamp. The EPR spectroscopy was recorded using a JES-FA200 (JEOL, Japan) EPR spectrometer. The temperature-dependent luminescence was measured under temperature control (THMS-600) in combination with a homemade PL measurement system consisting of a 365-nm NUVLED array as the excitation source and a micro-Raman spectrometer (Jobin-Yvon HR800) as the detector. A full set of 14 CRIs and the average CRI (Ra) values of the powders were measured using a homemade PL measurement system with a 365-nm UV light source (LA-410, full width at half maximum (FWHM): 5 nm, Japan) and a band-pass filter (Asahi spectra, Japan) as well as the same miniature fiber optic spectrometer with an integrated sphere. The electroluminescent (EL) spectrum and the CRI values of the commercial WLED were also measured using the same miniature spectrometer. The quantum yield (QY) was measured using a PL quantum-yield measurement system (C9920-02, Hamamatsu Photonics).
Results and Discussion
Figure 1 presents the structural differences between Sr5(PO4)3Cl (SPO_Cl) and Sr5(BO4)3Cl (SBO_Cl) and a coordinated polyhedron of anions/cations. SPO_Cl crystallizes as a hexagonal structure and has two cationic sites, referred to as Sr1 and Sr2. These sites reside in the 4f and 6h sites (Figure 1a)8. The coordination polyhedron of Sr1/O is composed of six O atoms, and the coordination polyhedron of Sr2/O is composed of two Cl and five O atoms. The P ions are connected with four O ions, forming the PO4 tetrahedron structure8. The crystallization of SBO_Cl in the orthorhombic structure is a complex three-dimensional framework of 7-, 8-, and 9-coordinate Sr-centered polyhedral and BO3 triangular planes (Figure 1b). There are four cationic sites, which are referred to as Sr1, Sr2, Sr3, and Sr4, that reside in the 4a, 8c, 4b, and 4b sites, respectively30. The B ions are surrounded by three O atoms forming a BO3 triangular plane with O-B-O bond angles near 120°.
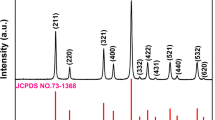
The calculated and experimental results as well as their differences in the XRD refinement of SPO_Cl are shown in Figure 2a. The initial structural mode was established by the standard crystallographic data of the SPO_Cl (ICSD#0993). The zero point error, scale factor, and background lattice and parameters were refined first followed by the profile parameters (i.e., W, V, U, X, and Y.). Finally, the structural parameters were refined. Based on the Finger-Cox-Jephcoat profile function model, the refinement of the low-angle asymmetry significantly improved the reliability of the pattern fitting. The final reliability factors for the complete pattern were Rwp = 8.46% and Rp = 5.46%. The refined lattice parameters were a = b = 9.872(5) Å, c = 7.187(9) Å, and V = 606.7(3) Å3. These results agree well with the results previously reported32.
(a) Observed (crosses) and calculated (solid lines) XRD patterns of the Rietveld refinement of the as-prepared Sr5(PO4)3Cl powder. (b) XRD patterns of the SPBO_Cl:0.04Eu2+ (0 ≤ x ≤ 0.5). As a reference, the standard XRD data for Sr5(PO4)3Cl is shown. (c) Magnified XRD patterns in the region between 30° and 32° for the SPBO_Cl:0.04Eu2+ (0 ≤ x ≤ 0.5). (d) Unit cell parameters of the SPBO_Cl:0.04Eu2+ (0 ≤ x ≤ 0.5) show a contraction in the lattice parameters a and b but only a slight increase in lattice parameter c.
Figure 2b shows the XRD patterns of the Sr5(PO4)3-x(BO3)xCl:0.04Eu2+ (0 ≤ x ≤ 0.5) powders. The XRD patterns of the Sr5(PO4)3-x(BO3)xCl:0.04Eu2+ (SPBO_Cl:0.04Eu2+) solid solution all maintain the characteristic pattern of the hexagonal structure of the SPO_Cl end member. These are consistent with literature values (Sr5(PO4)3Cl, PDF card No. 16-0666)33. No detectable XRD peaks corresponding to impurities or other phases were observed, indicating that solid solutions were obtained. When x > 0.5, some by-products appeared in the power sample due to the large mismatch (35%) of the ionic radii of P5+ (r = 0.17 Å, CN = 4) and B3+ (r = 0.11 Å, CN = 4). We therefore believe that the Sr5(PO4)3-x(BO3)xCl compounds for 0 ≤ x ≤ 0.5 were primarily solid solutions. The XRD peaks located at 30.3° and 31.4° correspond to the (211) and (300) crystal planes, respectively. These shift slightly to the higher scattering angle with an increase in the B3+ ion concentrations, whereas the XRD peak at 30.7° shifts to the lower scattering angle side (Figure 2c). The XRD peaks shift toward the higher angle side. This trend is also seen on almost all of the other diffractive peaks except for the XRD peaks corresponding to the (002), (102), (222), (213), and (004) crystal planes. These observations indicate the substitution of smaller B3+ (r = 0.11 Å, CN = 4), for P5+ (r = 0.17 Å, CN = 4) changes the lattice parameters of the host. As shown in Figure 2d, the calculated lattice constants a and b decrease linearly as x increases, whereas the lattice constant c is mostly constant. The simultaneous expansion and contraction of the unit cell in different directions suggests that the anionic polyhedron substitution of PO4 with BO3 causes a greater distortion of the host lattice end member.
The distortion of the host lattice has a significant effect on Eu2+ luminescence. As reported, Eu2+ occupied the Sr2 sites in the SPO_Cl host lattice more often than the Sr1 sites. Therefore, only one emission band in the blue spectral region was observed28,29. In our case, SPO_Cl:yEu2+ (0.02 ≤ y ≤ 0.1) exhibited an asymmetric blue emission at approximately 446 nm under excitation at 365 nm (see Supplementary Fig. S1). As the Eu2+ content increased, the luminous intensity was intensified and reached a maximum at y = 0.04 (see Supplementary Fig. S2). Herein, we consider that most Eu2+ ions occupy the Sr2 sites (446 nm) rather than Sr1 sites (462 nm) in the SPO_Cl host, which supports the findings of previous reports28,29.
Interestingly, an additional distinct emission peak at approximately 550 nm was observed under excitation at the same wavelength when doped with B3+ ions (see Supplementary Fig. S1). This observation suggests that the introduction of B3+ ions induces a distinct Eu2+ center that originates from the Eu2+ occupying a distorted crystal field environment. The emission spectra can be deconvoluted into three Gaussian components with peaks centered at approximately 446 and 462 nm (Eu(1)) and 550 nm (Eu(2)), as shown in Supplementary Fig. S1. We assign the higher-energy emission (446 and 462 nm) to Eu(1), which occupies Sr2/Sr1 sites with a weak crystal field. From the X-ray data measured here, it is difficult to determine which sites the induced Eu2+ ions can occupy. Therefore, we tentatively assigned the lower-energy emission (550 nm) to Eu(2) occupying the perturbed Sr2 with a strong crystal field. With an increase in the B3+-doped content, the QY values of the corresponding samples obviously decrease from 82% at x = 0 to 53% at x = 0.5 at an excitation of 365 nm, which should indicate the formation of more defects in the host lattice. Optimizing the synthesis process is expected to increase the QY.
Color tunability is very significant for phosphors because it facilitates WLED color tuning34. The normalized PL spectra (λex = 365 nm) of the SPBO_Cl:0.04Eu2+ powders (x = 0, 0.1, 0.2, 0.3, 0.4, and 0.5) are presented in Figure 3a. The relative intensity of the yellow to blue emission increases gradually with an increase in the B3+ content. This reaches a maximum at x = 0.5, whereas the intensity of the blue emission steadily decreases (Figure 3b). Therefore, a tunable color spectrum including white light can be obtained by combining the two emission bands located at approximately 446 and 550 nm in this SPBO_Cl:0.04Eu2+ solid-solution phosphors. The change in the emission ratio may be attributed to the higher number ratio of the Eu(2) relative to the Eu(1) sites. These are dependent on the structural variation in the solid-solution phosphor. Conversely, the energy transfer from the Eu(1) to Eu(2) sites also contributes to the change in the emission ratio. This will be shown and discussed in detail in the lifetime decay section.
(a) Normalized PL spectra of SPBO_Cl:0.04Eu2+ (0 ≤ x ≤ 0.5) under excitation at 365 nm. (b) The integrated PL intensity of the higher-energy emission and the lower-energy emission of SPBO_Cl:0.04Eu2+ as a function of the B3+ content (0 ≤ x ≤ 0.5) under excitation at 365 nm. (c), (d), and (e) the fluorescence microscope PL images for SPBO_Cl:0.04Eu2+ (x = 0, 0.2, and 0.5) under excitation at 365 nm.
Next, confocal fluorescence microscopy PL images were recorded using the 365-nm UV lamp excitation to demonstrate the PL characteristics of the phosphor particles. The emission color of these microcrystalline powders was visible to the naked eye and was tunable from blue to blue-greenish and ultimately to white luminescence (Figure 3c–3e). The calculated CIE color coordinates can be used to characterize the emission spectra (Figure 7 and Supplementary Table S1). These values range from (0.162, 0.062) at x = 0 to (0.327, 0.361) at x = 0.5. The color tones are in accordance with the results of the fluorescence microscopy PL images.
In addition, the FWHM of the PL spectra was quite broad for samples x ≥ 0.3 and covers a wide range of the visible spectrum. This is helpful for creating high CRI phosphor-converted WLEDs. For example, the composition at x = 0.5 can achieve greater CRI white-light versus commercial YAG:Ce3+-based WLEDs. This will be shown and discussed in Figure 8. A continuous redshift is also observed in the two emission bands with an increase in the B3+ content (see Supplementary Table S1). In our case, there are two possible causes for the PL redshift: (1) reabsorption; and (2) increased crystal field splitting of Eu2+. In reabsorption, the greater energy emission generally resonates with the lower energy part of the excitation spectra. This results in the partial reabsorption of the high-energy emission and a shift in the emission to the red. Herein, it is clear that the higher-energy emission overlaps the low energy PLE spectra (λem = 550 nm). The extent of the overlap becomes more prominent with an increase in the B3+ content. In the case of increased Eu2+ crystal fields, the lowest 5d energy level of Eu2+ is strongly influenced by the crystal field splitting. Therefore, the PL spectrum will redshift when the splitting of the Eu2+ 5d energy levels increases. As shown in the XRD section, the anionic polyhedron PO4 units are successively replaced with BO3 by increasing the B3+ content. This reduces the unit cell volume and decreases the Sr-O (Eu-O) bond length. A decreased Eu-O bond length possibly increases the crystal field splitting of the Eu2+ ions in the 5d energy level. This causes a continuous redshift in the emission spectra.
To validate this hypothesis, the EPR data were used to investigate the surroundings of the Eu2+ ions because the EPR signals from the Eu2+ ions are very sensitive to the area surrounding the Eu2+ ions (4f7, S = 7/2, L = 0). Figure 4 shows the EPR spectra of end member SPO_Cl and SPBO_Cl:0.04Eu2+ powders (x = 0, 0.2, 0.4, and 0.5), respectively. At x = 0, there is a very weak EPR signal for the SPO_Cl end member (no Eu) indicating that paramagnetic impurities exist in the host. These impurities may originate from sample defects. The weak EPR signal corresponding to the defects can be neglected compared with the EPR signal of the SPO_Cl:0.04Eu2+. Accordingly, the EPR signals of the SPO_Cl:0.04Eu2+ can be totally attributed to the unpaired electrons in the Eu2+. We consider that the local coordination environments of Eu2+ can be divided into two sections: with and without B5+ (x = 0 and x = 0.2–0.5, respectively). These sections yield different emissions, including Eu(1) at 446 nm and Eu(2) at 550 nm. In contrast, the difference in the EPR signals (indicated by *) is likely due to changes in the Eu2+ sites in the host lattice with the addition of B5+. This result indicates that the introduction of B5+ induces a distinct Eu2+ site that may occupy the perturbed Sr site in the host lattice. Similar results have also been reported in the literature.35 By increasing B5+ content, we found that the FWHM of the EPR spectra of SPBO_Cl:0.04Eu2+ (x = 0.2, 0.4, and 0.5) is gradually broadened. This result presents evidence that the introduction of B3+ significantly intensifies the interaction between Eu2+ centers and the host lattice36, leading to an increase in the energy-level splitting of Eu2+ 5d. This redshifts the PL spectra. In addition, the Stokes shift for both emission bands changes with an increase in the B3+ content, but this change is not significant (see Supplementary Table S1). Therefore, we consider the redshift of the PL spectra to be primarily due to reabsorption and increased crystal filed splitting of Eu2+. Of course, the contribution of the Stokes shift to the observed redshift cannot be ruled out.
The normalized PLE spectra for Sr5(PO4)3-x(BO3)xCl:0.04Eu2+ (x = 0, 0.1, 0.2, 0.3, 0.4, and 0.5) samples were obtained using the maxima value of each PL band. Monitoring the blue emission showed that the PLE spectrum has a broad band from 250 nm to 440 nm (Figure 5a). Similarly, when monitoring yellow emission, broad PLE spectra were observed in the range of 250–475 nm (Figure 5b). Both PLE spectra are ascribed to the 4f→5d transitions of Eu2+. Nevertheless, different spectral profiles for the two PLE bands suggest that there is a difference in the splitting of the 4f65d1 excited states for the two sites. This concurs with the PL and EPR results.
(a) Normalized PLE spectra of the SPBO_Cl:0.04Eu2+ (0 ≤ x ≤ 0.5) obtained by monitoring the higher-energy emission. (b) Normalized PLE spectra of the SPBO_Cl:0.04Eu2+ (0 ≤ x ≤ 0.5) obtained by monitoring the lower-energy emission. Additionally, the PL spectrum of Sr5(PO4)3Cl:0.04Eu2+ is also shown as a reference.
When increasing the B3+ content, the Eu2+ 5d bands in both PLE spectra shift to the lower-energy side along with profile changes. This is a consequence of the stronger crystal field splitting of the Eu2+ ions’ 5d energy level as discussed previously. In addition, the bandgaps (Eg) of SPO_Cl, SPBO_Cl, and SPBO_Cl:0.04Eu2+ are calculated from their reflectance spectra. The undoped sample shows no absorption in the wavelength range greater than 375 nm and is thus white, which agrees with the PLE results. The Eg of SPO_Cl is estimated to be 3.836 eV. The SPBO_Cl:0.04Eu2+ has a strong absorption from 250 nm to 475 nm, and the color turns light yellow (see Supplementary Figure S3). This result is attributed to the 4f–5d transitions at the Eu2+ center. The Eg of SPBO_Cl:0.04Eu2+ is estimated to be 2.850 eV. The PL spectra (λex = 365 nm) of SPO_Cl:0.04Eu2+ is also shown in Figure 5b as a reference. Obviously, there is a significant spectral overlap between the blue emission located at 446 nm (Eu(1)) and the PLE spectra monitored by the lower-energy emissions of Eu(2) from 410 nm to 470 nm. This result implies the possibility of energy transfer from the Eu(1) to the Eu(2) site.
To further investigate the dynamic luminescence process between the higher-energy emission sites and the lower-energy emission sites, the lifetime decay of the higher-energy emission sites in the SPBO_Cl:0.04Eu2+ powders (x = 0, 0.1, 0.3, and 0.5) was recorded (λex = 365 nm; Figure 6a). At x = 0, the decay curve of the higher-energy emission at 446 nm presents a single exponential decay behavior. However, the fluorescence decay of the higher-energy emission becomes faster with an increase in the B3+ content, and the decay curves are non-exponential. This suggests that the incorporation of B5+ ions changes the luminescence dynamic process of Eu2+. The average lifetime (τ) of these samples can be estimated using Equation (1)37:

(a) The lifetime decay curves for the Eu(1) in SPBO_Cl:0.04Eu2+ phosphors (x = 0, 0.1, 0.3, and 0.5) excited at 365 nm and monitored at 446 nm. The inset shows the calculated energy transfer efficiency (ηET) from Eu(1) to Eu(2) with a varying B3+ doping content. (b) The lifetime decay curves for Eu2+ in the SPBO_Cl:0.04Eu2+ (x = 0.1) phosphor excited at 275 nm and monitored at 448 and 564 nm. The lifetime decay curves were fitted using the double-exponential equation I(t) = I0 + A1exp(−t/τ1) + A2exp(−t/τ2).
where I(t) is the luminous intensity at time t. According to Equation (1), the average lifetimes of higher-energy emission are 0.56, 0.47, 0.39, and 0.32 μs for B3+ values of 0, 0.1, 0.3, and 0.5, respectively. The decrease in the lifetime of the higher-energy emission with an increase in the B3+ content strongly demonstrates an energy transfer from the higher-energy emission sites to the lower-energy emission sites. In this case, the calculated lifetime values corresponding to the higher-energy emission cannot be completely assigned to the 446-nm Eu(1) sites because the 446- and 462-nm emissions from the Eu(1) site are simultaneously recorded (Supplementary Fig. S1). With regard to the lifetime of the Eu(2) acceptor, an initial increasing trend should be observed if the energy transfer from the higher-energy emission to the lower-energy emission sites definitely occurs. Figure 6b shows the decay profile of the lower-energy emission (λem = 550 nm) for x = 0.1 samples at an excitation of 275 nm. This clearly shows that the decay profile is nonexponential with a prominent initial increasing trend. The calculated average lifetime using Equation (1) is 1.44 µs with a buildup characterized by a constant ∼0.37 µs. In contrast, the decay monitored the higher-energy emission (λem = 446 nm) is multi-exponential with the fastest component at ∼0.38 µs. The similarity in this time constant for the fastest decay component of the higher-energy emission as well as the increase in the lower-energy emission also suggests the occurrence of energy transfer from the higher-energy emission sites to the lower-energy emission sites.
The different decay profiles and lifetime values for the blue and yellow emissions further support the notion of the two emission bands from two distinct Eu2+ centers. In addition, the energy transfer efficiency (η) from the higher-energy emission to the lower-energy emission sites can be estimated using Equation (2)38,39:

where τso and τs are the lifetimes of the higher-energy emission sites in the absence and in the presence of the lower-energy emissions, respectively. As shown in the inset of Figure 6b, η increases gradually with an increase in the B3+ content. More precisely, the energy-transfer efficiencies, η, are calculated to be 0%, 16.9%, 26.7%, 31.2%, 41.7%, 42.1%, and 44.2% for SPBO_Cl:0.04Eu2+ when x = 0, 0.1, 0.2, 0.3, 0.4, 0.5, and 0.6, respectively.
To evaluate the potential application of an as-prepared sample, the PL spectrum of the SPBO_Cl:0.04Eu2+ phosphor (x = 0.5) at a 365-nm excitation is recorded and presented in Figure 8. White light can clearly be generated by combining the blue emission located at approximately 455 and the yellow emission at 550 nm. Interestingly, the white-light emission profile of the as-prepared sample is very similar to the emission profile of the commercial WLED (blue LED chip + Y3Al5O12:Ce3+ (YAG:Ce3+) yellow-emitting phosphor).
For comparison, the EL spectrum of the commercial WLED driven by a forward bias at 20 mA is also presented in Figure 8. The full set of 8 CRIs and the average CRI (Ra) of the as-prepared sample as well as the commercial WLEDs are listed in Supplementary Table S2, respectively. All 8 CRIs are greater than the YAG:Ce3+-based WLED. The as-prepared sample has a greater Ra value (∼91.0) versus the YAG:Ce3+-based WLED (∼80.2). Ra is widely used in the lighting community to evaluate the performance of artificial lighting including WLEDs40,41,42; however, a high Ra does not completely guarantee the good saturation of colors of illuminated objects43 because the Ra is calculated only using the first eight (R1–R8) values of the CIE from the recommended 14 test color samples43. Therefore, the special CRI R9 value, which denotes the color reproduction in the strong red region and is very important for biomedical and painting44,45,46,47,48, is critical for high-quality color renditions of the light source. Therefore, we found that the R9 of the as-prepared sample is greater (∼90.2) than the R9 of the current available lamp sources, e.g., halo-fluorescent lamps (∼–81)47, tri-phosphor fluorescent lamps (∼8)47, and commercial WLEDs (∼14.3) (see Supplementary Table S3). Compared with the YAG:Ce3+-based commercial WLED, the as-prepared sample has greater Ra and R9 values. This is consistent with the fact that there was greater number of blue-greenish (470–515 nm) and deep-red components in the emission spectrum and a wider spectral distribution (∼137 nm) at approximately 550 nm compared with the YAG:Ce3+ phosphor (∼104 nm).
Thus far, there have been many investigations of single Eu2+/Ce3+-activated white-light phosphors, e.g., (BaSrMg)(PO4)2:Eu2+ (Ra = 87)19, (Na1−xCax)(Sc1−xMgx)Si2O6:Eu2+ (Ra = 72)20, (Gd1−xSr2+x)AlO5-xFx:Eu2+ (Ra = 53)21, Sr2Ba(AlO4F)1−x(SiO5)x:Ce2+ (Ra = 70)22, (La0.075-xCe0.025Sr2+x)(Al1−xSixO5) (Ra = 70)23, and (Sr2.975Ce0.025)Al1−xSixO4+xF1−x (Ra = 69)24. However, indices greater than Ra = 91.0 and R9 = 90.2 have not yet been reported. These results indicate that this single-phased SPBO_Cl:0.04Eu2+ white-light phosphor is a promising candidate for NUVLED-excited devices for general illumination purposes.
Thermal stability is an important technological parameters for a WLED phosphor. The thermal quenching behavior of the SPBO_Cl:0.04Eu2+ phosphor (x = 0.5) in the temperature range of 20–200 °C under an excitation at 365 nm is shown in Supplementary Fig. S4. The emission intensity of Eu2+ decreased with an elevated temperature (Supplementary Fig. S4a). The emission intensity decreased to 50% of the initial values at 120 °C (Supplementary Fig. S4b). Furthermore, the thermal quenching temperatures for both emission bands of the SPBO_Cl:0.04Eu2+ phosphor were different (Supplementary Fig. S4c). The thermal quenching temperature for the yellow emission was 150 °C, whereas that for blue was 90 °C. Different thermal quenching temperatures for the blue and the yellow emissions may originate from the distinct Eu2+ ions with different rigid environments. As for the solid-solution white-light phosphor (x = 0.5), the thermally activated energy was calculated in terms of the Arrhenius equation I(T) ≍ I0[1+A exp (–ΔE/kBT)]–1 (where I0 is the initial intensity, I(T) is the intensity at a given temperature T, A is a constant, ΔE is the activation energy for the thermal quenching, and kB is Boltzmann’s constant). The large decrease in luminescence might be attributed to the smaller barrier energy (∼0.269 eV).
Conclusions
The Sr5(PO4)3-x(BO3)xCl:0.04Eu2+ phosphors demonstrate a continuously tunable emission and were obtained using a solid-solution method and a solid-state reaction. Based on the Rietveld and EPR analysis, substituting PO4 with BO3 anionic polyhedrons contracts and distorts the lattice of the end member Sr5(PO4)3Cl of the solid solution. This induces a new Eu2+ yellow emission at approximately 550 nm. The distinct Eu2+ center originates from the Eu2+, occupying a different crystal field site. Combining a blue emission near 446 nm and an induced yellow emission at 550 nm results in a tunable emission including white light via 365-nm excitation. Significantly, the white-light phosphor presents greater Ra and R9 values (91 and 90.2, respectively) relative to commercial YAG:Ce3+-based WLEDs (Ra = 80 and R9 = 14.3). This study provides an important shortcut for developing a high CRI white-light phosphor from single-Eu2+-doped compounds.
References
Yamamoto H . White LED phosphors: the next step. In: Jiang SB, Digonnet MJF, Glesener JW, Dries JC, editors. Proceedings of SPIE optical components and materials VII; 23 January 2010, Vol. 7598. San Francisco, CA: SPIE; 2010. pp 759808–759811.
Hartmann P, Pachler P, Payrer EL, Tasch S . Up and down: color conversion for solid-state lighting. In: Streubel KP, Jeon H, Tu LW, editors. Proceedings of SPIE light-emitting diodes: materials, devices, and applications for solid state lighting XIII; 24 January 2009, Vol. 7231. San Jose, CA: SPIE; 2009. p 72310X.
Li XF, Budai JD, Liu F, Howe JY, Zhang JH et al. New yellow Ba0.93Eu0.07Al2O4 phosphor for warm-white light-emitting diodes through single-emitting-center conversion. Light Sci Appl 2013; 2: e50 ; doi:10.1038/lsa.2013.6.
Shang MM, Li CX, Lin J . How to produce white light in a single-phase host? Chem Soc Rev 2014; 43: 1372–1386.
Hao ZD, Zhang JH, Zhang X, Sun XY, Luo YS et al. White light emitting diode by using α-Ca2P2O7:Eu2+, Mn2+ phosphor. Appl Phys Lett 2007; 90: 261113.
Chang CK, Chen TM . White light generation under violet-blue excitation from tunable green-to-red emitting Ca2MgSi2O7: Eu, Mn through energy transfer. Appl Phys Lett 2007; 90: 161901.
Wu WW, Xia ZG . Synthesis and color-tunable luminescence properties of Eu2+ and Mn2+-activated Ca3Mg3(PO4)4 phosphor for solid state lighting. RSC Adv 2013; 3: 6051–6057.
Chen X, Dai PP, Zhang XT, Li C, Lu S et al. A highly efficient white light (Sr3, Ca, Ba)(PO4)3Cl:Eu2+, Tb3+, Mn2+ phosphor via dual energy transfers for white light-emitting diodes. Inorg Chem 2014; 53: 3441–3448.
Lü W, Hao ZD, Zhang X, Luo YS, Wang XJ et al. Tunable full-color emitting BaMg2Al6Si9O30: Eu2+, Tb3+, Mn2+ phosphors based on energy transfer. Inorg Chem 2011; 50: 7846–7851.
Geng DL, Shang MM, Yang DM, Zhang Y, Cheng ZY et al. Green/green-yellow-emitting KSrGd(PO4)2: Ce3+, Tb3+/Mn2+ phosphors with high quantum efficiency for LEDs and FEDs. Dalton Trans 2012; 41: 14042–14045.
Jiao MM, Jia YC, Lü W, Lv WZ, Zhao Q et al. A single-phase white-emitting Ca2SrAl2O6:Ce3+, Li+, Mn2+ phosphor with energy transfer for UV-excited WLEDs. Dalton Trans 2014; 43: 3202–3209.
Li GG, Geng DL, Shang MM, Zhang Y, Peng C et al. Color tuning luminescence of Ce3+/Mn2+/Tb3+-triactivated Mg2Y8(SiO4)6O2 via energy transfer: potential single-phase white-light-emitting phosphors. J Phys Chem C 2011; 115: 21882–21892.
Huang CH, Chen TM . A novel single-composition trichromatic white-light Ca3Y(GaO)3(BO3)4:Ce3+, Mn2+, Tb3+ phosphor for UV-light emitting diodes. J Phys Chem C 2011; 115: 2349–2355.
Li GG, Zhang Y, Geng DL, Shang MM, Peng C et al. Single-composition trichromatic white-emitting Ca4Y6(SiO4)6O: Ce3+/Mn2+/Tb3+ phosphor: luminescence and energy transfer. ACS Appl Mater Interfaces 2012; 4: 296–305.
Setlur AA, Shiang JJ, Happek U . Eu2+–Mn2+ phosphor saturation in 5 mm light emitting diode lamps. Appl Phys Lett 2008; 92: 081104.
Happek U, Setlur AA, Shang JJ . Inverse bottleneck in Eu2+–Mn2+ energy transfer. J Lumin 2009; 129: 1459–1463.
Yang FL, An W, Zhuang WD, Tian GS, Jing XP . Energy transfer from Eu2+ to Mn2+ in M5(PO4)3Cl (M = Ca, Sr). J Lumin 2014; 146: 269–274.
Yang FL, An W, Wei HW, Chen GT, Zhuang WD et al. Comparison of the PL properties of Eu2+, Mn2+ co-doped M5(PO4)3Cl (M = Ca, Sr, Ba). J Alloys Compd 2014; 590: 535–540.
Wu ZC, Liu J, Hou WG, Xua J, Gong ML . A new single-host white-light-emitting BaSrMg(PO4)2:Eu2+ phosphor for white-light-emitting diodes. J Alloys Compd 2010; 498: 139–142.
Xia ZG, Zhang YY, Molokeev MS, Atuchin VV, Luo Y . Linear structural evolution induced tunable photoluminescence in clinopyroxene solid-solution phosphors. Sci Rep 2013; 3: 3310.
Im WB, Fourré Y, Brinkley S, Sonoda J, Nakamura SJ et al. Substitution of oxygen by fluorine in the GdSr2AlO5:Ce3+ phosphors: Gd1−xSr2+xAlO5-xFx solid-solutions for solid state white lighting. Opt Express 2009, 17: 22673–22679.
Denault KA, Georege NC, Paden SR, Brinkley S, Mikhailovsky AA et al. A green-yellow emitting oxyfluoride solid-solution phosphor Sr2Ba(AlO4F)1−x(SiO5)x:Ce3+ for thermally stable, high color rendition solid state white lighting. J Mater Chem 2012; 22: 18204–18213.
Im WB, Fellows NN, DenBaars SP, Seshadri R . La1−x−0.025Ce0.025Sr2+xAl1−-xSixO5 solid solutions as tunable yellow phosphors for solid state white lighting. J Mater Chem 2009; 19: 1325–1330.
Im WB, George N, Kurzman J, Brinkley S, Mikhailovsky A et al. Efficient and color-tunable oxyfluoride solid solution phosphors for solid-state white lighting. Adv Mater 2011; 23: 2300–2305.
Bachmann V, Ronda C, Oeckler O, Schnick WG, Meijerink A . Color point tuning for (Sr, Ca, Ba)Si2O2N2:Eu2+ for white light LEDs. Chem Mater 2009; 21: 316–325.
Song YH, You HP, Yang M, Zheng YH, Liu K et al. Facile synthesis and luminescence of Sr5(PO4)3Cl:Eu2+ nanorod bundles via a hydrothermal route. Inorg Chem 2010; 49: 1674–1678.
Guo C, Luan L, Ding X, Zhang F, Shi FG et al. Luminescent properties of Sr5(PO4)3Cl:Eu2+, Mn2+ as a potential phosphor for UV-LED based white LEDs. Appl Phys B 2009; 95: 779–785.
Zeng Q, Liang HB, Zhang GB, Birowosuto MD, Tian ZF et al. Luminescence of Ce3+ activated fluoro-apatites M5(PO4)3F (M = Ca, Sr, Ba) under VUV–UV and x-ray excitation. J Phys Condens Matter 2006; 18: 9549–9560.
Gruber JB, Zandi B, Merkle L . Crystal-field splitting of energy levels of rare-earth ions Dy3+4f9 and Yb3+, 4f13 in M(II) sites in the fluorapatite crystal Sr5(PO4)3F. J Appl Phys 1998; 83: 1009–1017.
Alekel T, Keszler DA . Structure of Sr5(BO3)3Cl. Acta Cryst C 1992; 48: 1382–1386.
Zhang QH, Wang J, Yu RJ, Zhang M, Su Q . Luminescence properties of Eu2+ ions in Sr5(BO3)3Cl. Electrochem Solid-State Lett 2008; 11: H335–H337.
Larson AC, Von Dreele RB . General structure analysis system (GSAS). Report LAUR 86-748; Los Alamos National Laboratory, University of California, Los Alamos, NM; 2004.
Nagpure IM, Pitale SS, Coetsee E, Ntwaeaborwa OM, Terblans JJ et al. Lattice site dependent cathodoluminescence behavior and surface chemical changes in a Sr5(PO4)3F host. Phys B 2012; 407: 1505–1508.
McKittrick J, Hannah ME, Piquette A, Han JK, Choi JI et al. Phosphor selection considerations for near-UV LED solid state lighting. ECS J Solid State Sci Technol 2013; 2: R3119–R3131.
Im WB, Kim YI, Yoo HS, Jeon DY . Luminescent and structural properties of (Sr1−x, Bax)3MgSi2O8:Eu2+: effects of Ba content on the Eu2+ site preference for thermal stability. Inorg Chem 2009; 48: 557–564.
Xu YZ . Applied electron magnetic resonance spectroscopy. Beijing: Science Press; 2008. pp 163–164.
Guo N, Song YH, You HP, Jia G, Yang M et al. Optical properties and energy transfer of NaCaPO4:Ce3+, Tb3+ phosphors for potential application in light-emitting diodes. Eur J Inorg Chem 2010; 29: 4636−4642.
Lahoz F, Martín IR, Méndez-Ramos J, Núñez P . Dopant distribution in a Tm3+-Yb3+ codoped silica based glass ceramic: an infrared-laser induced upconversion study. J Chem Phys 2004; 120: 6180–6190.
Paulose PI, Jose G, Thomas V, Unnikrishnan NV, Warrier MKR . Sensitized fluorescence of Ce3+/Mn2+ system in phosphate glass. J Phys Chem Solids 2003; 64: 841–846.
Huang CH, Chen TM . Novel yellow-emitting Sr8MgLn(PO4)7:Eu2+ (Ln = Y, La) phosphors for applications in white LEDs with excellent color rendering index. Inorg Chem 2011; 50: 5725–5730.
Jang HS, Yang HS, Kim SW, Han JY, Lee SG et al. White light-emitting diodes with excellent color rendering based on organically capped CdSe quantum dots and Sr3SiO5:Ce3+ phosphors. Adv Mater 2008; 20: 2696–2702.
Kimura N, Sakuma K, Hirafune S, Asano K, Hirosaki N et al. Extra high color rendering white light-emitting diode lamps using oxynitride and nitride phosphors excited by blue light-emitting diode. Appl Phys Lett 2007; 90: 051109.
Oh JH, Yang SJ, Do YR . Healthy, natural, efficient and tunable lighting: four-package white LEDs for optimizing the circadian effect, color quality and vision performance. Light Sci Appl 2014; 3: e141 ; doi:10.1038/lsa.2014.22.
Kwon KH, Im WB, Jang HS, Yoo HS, Jeon DY . Luminescence properties and energy transfer of site-sensitive Ca6-x-yMgx-z(PO4)4:Euy2+, Mnz2+ phosphors and their application to near-UV LED-based white LEDs. Inorg Chem 2009; 48: 11525–11532.
Wang XB, Yan XS, Li WW, Sun K . Doped quantum dots for white-light-emitting diodes without reabsorption of multiphase phosphors. Adv Mater 2012; 24: 2742–2747.
Fukui T, Kamon K, Takeshita J, Hayashi H, Miyachi T et al. Superior illuminant characteristics of color rendering and luminous efficacy in multilayered phosphor conversion white light sources excited by near-ultraviolet light-emitting diodes. Jpn J Appl Phys 2009; 48: 112101.
Narukawa Y, Ichikawa M, Sanga D, Sano M, Mukai T . White light emitting diodes with super-high luminous efficacy. J Phys D Appl Phys 2010; 43: 354002.
Narukawa Y, Narita J, Sakamoto T, Yamada T, Narimatsu H et al. Recent progress of high efficiency white LEDs. Phys Status Solidi A 2007; 204: 2087–2093.
Acknowledgements
The work was supported by the National Natural Science Foundations of China (Grant No. 51302034, 11304035, 51372036, 31271442, and 91233204), the National Basic Research Program (2012CB933703), the Fundamental Research Funds for the Central Universities (Grant No. 12QNJJ007, 12SSXM001), and the International Science & Technology Cooperation Program of China (2013DFG50150), and 111 project (No. B13013). We appreciate the help of Prof. Guodong Li of Jilin University in performing the EPR measurements.
Note: Accepted article preview online 6 September 2015
Author information
Authors and Affiliations
Corresponding authors
Additional information
Note: Supplementary information for this article can be found on the Light: Science & Applications' website.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 Unported License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Dai, PP., Li, C., Zhang, XT. et al. A single Eu2+-activated high-color-rendering oxychloride white-light phosphor for white-light-emitting diodes. Light Sci Appl 5, e16024 (2016). https://doi.org/10.1038/lsa.2016.24
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/lsa.2016.24
Keywords
This article is cited by
-
Thermally stable double perovskite Ca2YTaO6: Tb3+, Eu3+ phosphor for warm white LEDs
Journal of Materials Science: Materials in Electronics (2024)
-
Single-phase white-emitting phosphors Ba3Bi(PO4)3:Dy3+, Eu3+ with tunable correlated color temperature and high thermal stability towards light emitting applications
Journal of Materials Science: Materials in Electronics (2021)
-
Effect of the Hole Injection Layer Conductivity on the Performance of Polymer Light-Emitting Diodes
Electronic Materials Letters (2021)
-
Strategies to approach high performance in Cr3+-doped phosphors for high-power NIR-LED light sources
Light: Science & Applications (2020)
-
Undoped highly efficient green and white TADF-OLEDs developed by DMAC-BP: manufacturing available via interface engineering
Journal of Materials Science: Materials in Electronics (2020)