Abstract
The E3 ubiquitin ligase (E3) WWP1 is an oncogenic factor implicated in the maintenance of different types of epithelial cancers. The role of WW domain-containing E3 ubiquitin protein ligase 1 (WWP1) in haematological neoplasms remains unknown. Acute myeloid leukaemia (AML) is characterized by the expansion of malignant myeloid cells blocked at different stages of differentiation. Here we report that the expression of WWP1 is significantly augmented in a large cohort of primary AML patients and in AML cell lines, compared with haematopoietic cells from healthy donors. We show that WWP1 inactivation severely impairs the growth of primary AML blasts and cell lines in vitro. In vivo, we observed a reduced leukaemogenic potential of WWP1-depleted AML cells upon transplantation into immunocompromised mice. Mechanistically, WWP1 inactivation induces the accumulation of its protein substrate p27Kip1, which ultimately contributes to G0/G1 cell cycle arrest of AML blasts. In addition, WWP1 depletion triggers the autophagy signalling and reduces survival of leukaemic cells. Collectively, our findings provide molecular insights into the anti-cancer potential of WWP1 inhibition, suggesting that this E3 is a promising biomarker and druggable target in AML.
Similar content being viewed by others
Introduction
The E3 ubiquitin ligase WW domain-containing E3 ubiquitin protein ligase 1 (WWP1) is an oncogenic factor that has been implicated in the maintenance and progression of different types of epithelial cancers.1, 2, 3, 4, 5 WWP1 is frequently amplified and/or overexpressed in these tumours and its deregulation is tightly associated with unfavourable prognosis.3, 4, 5, 6 Mechanistically, WWP1 controls protein stability and/or function of a number of protein substrates with tumour suppressive activities, such as p27Kip1, p53 and SMADs.7, 8, 9, 10 As a result, WWP1 depletion promotes cell cycle arrest and induces apoptosis.2, 3, 10 Although WWP1 has been extensively studied in epithelial neoplasms, the association of WWP1 with haematological malignancies remains to be established.
Acute myeloid leukaemia (AML) is a highly heterogeneous haematological disorder, characterized by the clonal expansion of leukaemic progenitors arrested at various stages of myeloid differentiation and by the progressive accumulation of multiple genetic, epigenetic and chromosomal alterations.11 Prognosis of patients with AML is strongly influenced by the type of chromosomal or genetic alteration, as well by changes in gene expression.12, 13 Approximately one-third of AML patients do not achieve complete remission in response to chemotherapy and, even when complete remission occurs, about 70% of patients relapse within 5 years. Thus, there is urgent need to identify new biomarkers for patient stratification and new therapeutic targets.
Manipulating autophagy represents a novel opportunity of cancer therapy for some AML clinical subtypes. In acute promyelocytic leukaemia (APL), in which the t(15;17) translocation fuses the promyelocytic leukaemia (Pml) with the retinoic receptor α (RARα) gene, autophagy elicited by all-trans retinoic acid (ATRA) or arsenic trioxide (As2O3) contributes to the disposal of leukaemic cells. This is achieved through autophagy-mediated degradation of the PML-RARα fusion oncoprotein, which in turn restores RARα-dependent transcription and myeloid differentiation of blasts. Similarly, bortezomib promotes cytotoxicity of AML cells bearing internal tandem duplication mutations of the Fms-like tyrosine kinase 3 (FLT3-ITD), through autophagy-dependent degradation of the FLT3-ITD protein.19
We report here that WWP1 is overexpressed in AMLs, where it limits the stability of p27Kip and the autophagy signalling, thus contributing to sustain AML growth in vitro and in vivo.
Materials and methods
Clinical samples
Leukaemic samples were obtained from bone marrow aspirates of patients diagnosed with de novo AML at the Haematology Unit of the Department of Biomedicine and Prevention of the University of Tor Vergata and from Institute of Hematology L. e A. Seràgnoli of the University of Bologna. Informed consent was obtained from all patients according to the Declaration of Helsinki and the study was approved by the Institutional Review Boards. All human participants gave written informed consent.
Control bone marrow mononuclear cells (BMMNCs) were obtained from adult lymphoma patients staging BM with no evidence of haematological malignant cells. CD34+ cells were isolated from BM or peripheral blood (PB) of healthy donor individuals. Donors of PB were not subjected to granulocyte colony-stimulating factor-induced stem cell mobilization. BMMNCs and PBMNCs were prepared by Ficoll-Hypaque gradient centrifugation. CD34+ cells were purified by means of the MACS CD34 microbead kit (Miltenyi Biotec, Bergisch Gladbach, Germany).
Knockdown experiments
The pLKO.1 plasmid vectors (Sigma-Aldrich, St Louis, MO, USA) expressing the short hairpin RNAs are detailed in Supplementary Materials and Methods. An off-target scrambled vector was used as a control. AML cells and primary leukaemic samples were spin inoculated with recombinant lentiviruses (multiplicity of infection=10) and subjected to two spin infection cycles for 2 days, in the presence of 8 μg/ml polybrene. Transduced cells were selected with 1 μg/ml puromycin for three days, before experiments.
Overexpression studies
U937 cells were transduced with the WWP1-expressing pMYs-IRES-GFP retroviral construct that was packaged using the Phoenix amphotropic cell line. The collected retrovirus was subsequently used to transduce cells by spinoculation (two spin infection cycles for 2 days) in the presence of 8 μg/ml polybrene. Green fluorescent protein-transduced cells were isolated by fluorescence-activated cell sorting.
Real-time PCR
Primer sequences and conditions used for reverse transcriptase-quantitative PCR are described in Supplementary Materials and Methods. The results were normalized to human 18s expression. For the expression profile of WWP1 in AML patients, data were normalized by the geometric mean of the level of expression of three housekeeping genes (18s, Tbp and Ubc).
Western blotting
Western blotting conditions and antibodies are detailed in Supplementary Materials and Methods.
Generation of mCherry-EGFP-LC3-expressing NB4 and confocal microscopy
mCherry-EGFP-LC3 was stably expressed in NB4 from the pLVO-puro lentiviral construct.20 For WWP1 knockdown experiments, stably transduced cells were infected as described above. Cells were imaged using a confocal microscope (model TCS SP2; Leica, Wetzlar, Germany) and using LCS 3D software (Leica). Quantification of the percentage of cells displaying cherry-LC3 puncta was determined by analysing a minimum of 400 LC3-expressing cells per each condition.
Animal studies
Experiments involving mice were performed according the Italian guidelines and after approval of the Institutional Review Board, in agreement with the guidelines reported in Workman et al.21 Non-irradiated NOD-scid IL2rγnull mice were transplanted with AML cells (1 × 106 cells/mouse) via intravenous injection. At signs of disease mice were killed and infiltrated organs collected for further analysis.
Statistical analysis
Statistical evaluation was determined by a two-tailed t-test and values are expressed as mean±s.d. Differences were considered statistically significant at P<0.05. The analysis on public data sets is based upon data generated by The Cancer Genome Atlas Research Network (http://cancergenome.nih.gov/) and is described in details in the Supplementary Materials and Methods section.
Results
WWP1 is aberrantly expressed in AML patients
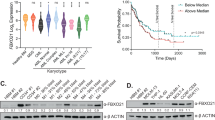
To investigate the clinical relevance of WWP1 in AML, we assessed whether its expression is altered in de novo AML patients. As indicated by gene expression profiling of haematopoietic cells (BloodSpot at www.bloodspot.eu), we observed a significant downregulation of WWP1 expression during myelopoiesis (mean fold=2, P=0.019 for PBMNCs; mean fold=6.8, P<0.0001 for BMMNCs as compared with CD34+ cells). In contrast, considering as a threshold the mean level of expression of WWP1 in the control CD34+ samples (mean 0.75), we scored overexpression of WWP1 in 77% (136/177) of the AML patients of our cohort (mean 2.45, P=0.0048; Figure 1a). WWP1 was equally overexpressed across different AML subtypes, with the exception of inv(16) AMLs (Figure 1a). In addition, we collected RNA-sequencing expression data from The Cancer Genome Atlas on 179 AML samples12 and used Genotype-Tissue Expression project data for healthy controls. Consistently, analysis of the The Cancer Genome Atlas data sets revealed significant WWP1 overexpression in all AML subtypes, except for AMLs carrying the t(8;21) (Figure 1b).
AML cells display high levels of WWP1. (a) WWP1 gene expression was assessed in newly diagnosed AML patients. BM-derived CD34+ and PBMNCs from healthy donors, and BMMNCs obtained from lymphoma patients without evidence of malignant tumour cells in their bone marrow were used as controls. Data are expressed as fold change of the average expression level of WWP1 in the CD34+ samples. P-values are calculated on the differences in WWP1 expression observed between AML patients and the control CD34+ population. *P⩽0.007, **P⩽0.04, ***P⩽0.02 and ****P⩽0.003. (b) WWP1 gene expression data (RNA-sequencing (RNA-seq)) for AML patients extracted from the TGCA database were compared with RNA-seq data from normal blood-derived samples obtained from the Genotype-Tissue Expression (GTEx) database. *P<0.0001. (c, d) WWP1 protein levels in primary samples (c) and AML cell lines (d). In d, both blots were cut vertically and juxtaposed to remove empty lanes.
We analysed WWP1 protein levels in primary leukaemic samples (patient characteristics in Supplementary Table S1) and in a panel of human AML cell lines representative of different AML FAB subtypes. WWP1 protein levels are almost undetectable in all control samples (CD34+ cells, PBMNCs and BMMNCs), whereas the amounts of WWP1 are increased, at different extent, in the majority of the AMLs analysed (Figures 1c and d, and Supplementary Figure S1a and b).
WWP1 silencing inhibits AML cell growth and delays leukaemogenesis
To dissect the role of WWP1 in leukaemogenesis, we inactivated the expression of WWP1 in human AML cell lines and primary samples. Short hairpin RNA-mediated gene silencing of WWP1 impaired growth of a panel of AML cell lines representing all FAB subtypes (Figurse 2a–e and Supplementary Figure S2a–f) and of primary cultures of AML blasts (Figures 2f–h and Supplementary Figure S2g–i). Although WWP1 silencing reduced the clonogenic ability of human CD34+ cells, it did not influence short-term proliferation and clonogenicity of normal murine haematopoietic stem-progenitors cells (Supplementary Figures S2j–l).
WWP1 inactivation impairs growth of AML cells in vitro and their leukaemogenic potential in vivo. (a–h) Growth curves of AML cell lines (a–e) and primary AML blasts (f–h) expressing scrambled control (sh-Scr) or WWP1 (sh-WWP1) short hairpin RNAs (shRNAs). Data are expressed as mean±s.d. and are representative of two individual experiments *P<0.05. (i) Growth curves of U937 cells infected with empty vector (EV) or WWP1-expressing lentiviruses. Data are representative of two individual experiments. (j) Overall survival of NSG mice transplanted with NB4 (left) and HL60 (right) cells transduced with sh-Scr and two sh-WWP1 RNAs (#395; #544). The plots combined data of four and three individual experiments for NB4 and HL60 cells, respectively. The silencing efficiency for each experimental replicate is shown in Supplementary Figure S3a and b.
To exclude off-target effects, we tested multiple WWP1 short hairpin RNAs and found that all were able to suppress growth of leukaemic cells, at an extent that mirrored their silencing efficiency (Supplementary Figure S2m and n). Similarly, Heclin, a small molecule inhibitor of the HECT E3s,22 inhibited growth of leukaemic blasts in a dose-dependent manner (Supplementary Figure S2o and p). Notably, ectopic expression of WWP1 accelerated growth (Figure 2i) in cells expressing almost undetectable amounts of endogenous WWP1 (U937 cells) (Figure 1d and Supplementary Figure S1b), demonstrating that WWP1 overexpression confers a proliferative advantage to AML cells.
We tested whether WWP1 depletion impairs human AML progression in vivo by using NOD-scid IL2rγnull (NSG) xenotrasplantation models. WWP1 depletion significantly increased survival of xenografts injected with either NB4 (FAB-M3) or HL60 (FAB-M2) cells (P<0.0001; Figure 2jand Supplementary Figure S3a and b). Reduced expression of WWP1 also diminished leukaemia penetrance from 100% (sh-Scr) to 93% and 80% (sh-WWP1) for the NB4 and HL60 transplants, respectively. The AML xenografts maintained WWP1 downregulation during tumour growth, although at a different extent (Supplementary Figure S3c and d).
We also determined the ability of WWP1 to influence the progression in vivo of a primary human AML (FAB-M4). We observed a consistent reduced ability of WWP1-depleted blasts to engraft in the recipients over time (Supplementary Figure S3e). The survival of mice transplanted with WWP1-depleted blasts was improved, as compared with control animals (Supplementary Figure S3f). However, the increase in survival did not reach statistical significance, probably due to the modest efficiency of WWP1 silencing in primary AML blasts (Supplementary Figure S3f upper panel). Notably, growing AML blasts re-expressed basal levels of WWP1 (Supplementary Figure S3g), suggesting that reduced amounts of WWP1 are quickly counter selected during in vivo growth.
Inactivation of WWP1 inhibits cell cycle progression and decreases survival of AML cells
Following WWP1 depletion, AML cells undergo G0/G1 growth arrest (Figure 3a upper panel and Supplementary Figure S4a). Consistently, ectopic expression of WWP1 increased the percentage of cycling leukaemic cells (Figure 3b). Cell cycle arrest of WWP1-depleted cells was preceded by the accumulation of its protein substrate p27Kip1 (7) (Figure 3a lower panels). p27Kip1 expression was not regulated transcriptionally upon WWP1 inactivation (Supplementary Figure S4b and data not shown). The early kinetics of p27Kip1 accumulation (Supplementary Figure S4c) suggests that this is not a consequence of the cell cycle arrest. However, inactivation of p27 expression in WWP1-depleted AML cells was unable to revert the inhibitory effect exerted by WWP1 downregulation on leukaemia growth (Supplementary Figure S4d). Indeed, in cells co-expressing short hairpin RNAs against p27Kip1 and WWP1, although p27Kip1 mRNA levels were reduced, the accumulation of p27Kip1 was still occurring at protein level, as a result of reduced proteasomal degradation by WWP1 (Figure 3c). Together, these findings imply that WWP1 loss induces cell cycle arrest by promoting posttranslational stabilization of p27Kip. Consistent with the impairment of cell cycle progression, we observed downregulation of Myc and induction of p21Waf1/Cip1 in WWP1-depleted cells (Figure 3d).
Knockdown of WWP1 induces cell cycle arrest and decreases viability of AML cell lines. (a) The cell cycle distribution was assessed by fluorescence-activated cell sorting (FACS) analysis of BrdU/propidium iodide (PI)-labelled sh-Scr and sh-WWP1 AML cells. Cells were analysed four days post-infection (upper panel). Data are representative of two independent experiments. p27Kip1 protein levels were analysed by IB 3 days post infection (lower panels). Blots are representative of three individual experiments. (b) Control (EV) and WWP1 overexpressing U937 cells were pulse-labelled with BrdU and the cell cycle profile was analysed as above. (c) WWP1 transcript and protein levels were analysed in NB4 cells expressing Scr, WWP1, p27Kip1 or both p27 Kip1/WWP1 short hairpin RNAs (shRNAs). Data are representative of two individual experiments. (d) IB of p21Waf1/Cip1 and Myc protein levels in sh-Scr and sh-WWP1 NB4 cells. Cells were harvested 4 days post infection. (e) Cell death was assessed in AML cell lines four days post infection. Apoptosis was quantified by monitoring the percentage of hypodiploid (sub-G1) cells (black bars). The percentage of necrotic cells was evaluated using the PI labelling (white bars). Data are representative of two individual experiments.
Previous studies have demonstrated that deficiency of WWP1 elicits apoptosis in epithelial tumour cells.2, 3, 23 Silencing of WWP1 decreased cell viability of all AML cell lines analysed, as assessed by loss of plasma membrane integrity (Figure 3e). However, WWP1 inactivation induced apoptotic cell death only in some AML cell lines (Figure 3e and Supplementary Figure S4e–j).
WWP1 downregulation increases the autophagic flux of AML cells
Given that persistent or excessive autophagic activation causes cell death with or without hallmarks of apoposis,24, 25, 26 we sought to determine whether WWP1 depletion might induce autophagy in leukaemic cells. At early time points preceding the onset of cell death, WWP1 depletion stimulated the conversion of cytosolic LC3-I into the lipid-bound LC3-II form (Figures 4a and b, and Supplementary Figure S5a), a marker of autophagosome membrane formation.27 Concomitantly, upon WWP1 silencing, we observed accumulation of the autophagy-associated protein ATG7 (Figures 4b and c). Reduced levels of the autophagic target p62/SQSTM1 in WWP1-depleted cells indicated an increased autophagic turnover (Figure 4b). Other components of the autophagic machinery were not significantly affected by WWP1 loss (Figure 4b and Supplementary Figure S5c).
WWP1 depletion activates autophagy in AML cells. (a) Autophagy was assessed in sh-Scr and sh-WWP1 AML cell lines by evaluating LC3-I to LC3-II conversion. Cells were collected four days post infection. A representative blot from two independent experimental replicates is shown. (b) IB analysis of LC3-II, ATG7, Beclin-1 and p62/SQSTM1 in sh-Scr and sh-WWP1 NB4 cells. (c) LC3 lipidation and ATG7 protein levels were analysed over time by IB. (d) Confocal images of fluorescent puncta in mCherry-EGFP-LC3 NB4 cells expressing sh-Scr or sh-WWP1 (#544) RNAs. Representative pictures from three independently performed experiments are shown. Baf-A1-treated cells (left panels) are shown as a control of autophagy inhibition. Bars are equal to 10 μM. (e) Autophagy was quantified in sh-Scr and three sh-WWP1 (#395, #544 and #488) samples, as the percentage of cells positive for Cherry-LC3 puncta (upper panel) and as average number of Cherry-LC3 puncta per cell (lower panel).
LC3-II accumulation upon WWP1 inactivation was further enhanced in the presence of the lysosomal inhibitor Bafilomycin A27 (Figure 4c and Supplementary Figure S5b), consistent with an active autophagic flux rather than a defect in autophagosome–lysosome fusion. To definitely distinguish whether autophagosome formation upon WWP1 depletion is due to induction of autophagic flux or rather to a block in downstream steps, we generated NB4 cells that stably express mCherry-EGFP-LC3. Upon exposure to the acidic environment of autolysosomes, the green fluorescent protein fluorescence is readily quenched and lost. Thus, an increase in red LC3 puncta reflects active autophagic flux.20 Following WWP1 inactivation, we observed an increased number of cells with Cherry-LC3 puncta as well as of Cherry-LC3 foci per cell (Figures 4d and e, and Supplementary Figure S5d).
WWP1 inactivation induces differentiation of AML cells by promoting autophagic degradation of oncogenic proteins
As WWP1 inactivation determines G0/G1 cell cycle arrest, we next examined whether WWP1 influences leukaemic cell differentiation. We monitored the effect of WWP1 silencing on the maturation of AML cell lines endowed with different differentiation competence in response to ATRA (Supplementary Figure S6a). We observed an early upregulation of CD11b expression and morphological features of maturing granulocytes only in NB4 and MV4-11 cells (Figure 5a and Supplementary Figure S6b–e, and data not shown), whereas CD14 expression was unaffected in all cell lines tested (data not shown). Notably, we did not detect features of myeloid maturation in the other AML cell lines even at later time points (data not shown). The absence of a correlation between the differentiation potential (Supplementary Figure S6a) of AML cells and the effects of WWP1 ablation on their maturation (Figure 5a) led us to further explore the mechanisms underlying NB4 and MV4-11 differentiation in response to WWP1 inactivation. Autophagy has been linked to differentiation of AML cells. Specifically, autophagy is a relevant pathway in the degradation of the PML-RARα oncoprotein and subsequent induction of granulocyte maturation.14, 15, 16, 17, 18 Consistently, we found a decrease in PML-RARα protein levels in WWP1-depleted NB4 cells (Figure 5b and Supplementary Figure S6f), whereas PML-RARα transcription was unaffected (Supplementary Figure S6g). Downregulation of WWP1 reduced the oncoprotein levels also in U937-PR9 cells (Figure 5c), which conditionally express PML-RARα.28 Destabilization of PML-RARα in WWP1-depleted NB4 cells resulted in a partial restoration of retinoid target-gene expression, including genes involved in granulocytic differentiation (Figure 5d). Notably, the NB4.007/6 ATRA-resistant subclone (Supplementary Figure S6a), in which PML-RARα is constitutively degraded through the proteasome,29 does not undergo differentiation upon WWP1 inactivation (Figure 5a). However, WWP1 depletion induces autophagy (Figure 4a) and inhibits growth (Supplementary Figure S2f) of NB4.007/6 cells. All together, these findings demonstrate that, in NB4 cells, loss of WWP1 leads to autophagy-dependent degradation of PML-RARα, thus relieving the differentiation block of APL blasts.
WWP1 controls granulocytic differentiation of AML cells by regulating the levels of autophagy sensitive oncoproteins. (a) Differentiation of AML cell lines was monitored by evaluating the expression of CD11b following WWP1 depletion. CD11b was analysed by fluorescence-activated cell sorting (FACS) 4 days post infection. Data are representative of two individual experiments. (b) IB analysis of PML-RARα and LC3 protein levels in sh-Scr and sh-WWP1 NB4 cells. Cells were collected 4 days post infection. Blot is representative of four individual experiments. (c) sh-Scr and sh-WWP1 U937-PR9 cells were incubated with 100 μmol/l Zn++ for 16 h to induce PML-RARα expression. Samples were processed for IB analysis of PML-RARα. (d) Reverse transcriptase-quantitative PCR (RT-qPCR) analysis of PML-RARα target genes in sh-Scr and sh-WWP1 NB4 cells. Cells were collected 4 days post infection. Data are presented as mean±s.d. and are representative of three independent experiments. (e) IB analysis of FLT3 and LC3 in sh-Scr and sh-WWP1 MV4-11 cells. Cells were collected 4 days post infection. Blot is representative of two individual experiments. (f) IB analysis of WWP1 protein levels in NB4 cells treated with increasing doses of ATRA for 3 days. (g) Analysis of WWP1 protein levels in sh-Scr and sh-WWP1 NB4 cells exposed to 10−6 M ATRA for 24 h and then incubated with MG132 for 1 h before collection. Blots are representative of three individual experiments. (h) Schematic model depicting the outcome of WWP1 loss in AML blasts. Depletion of WWP1 in AML cells would prevent leukaemia growth by abnormally accumulating p27Kip1 and ultimately blocking cell cycle entry. By inducing autophagy, WWP1 loss would reduce survival of AML cells by either autophagic (necrosis) or apoptotic cell death. Alternatively and independently of autophagy activation, WWP1-depleted AML cells could undergo apoptosis, as a result of loss of the anti-apoptotic activity of the E3. In AML cells expressing oncoproteins, such as PML-RARα and FLT3-ITD, which are subjected to autophagy-mediated degradation, WWP1 inactivation would promote their disposal and eventually contribute to granulocyte maturation or apoptotic cell death.
Similarly, autophagy induction is responsible for degradation of the FL3-ITD mutant protein,19 which contributes to the myeloid differentiation blockage of AML blasts in FLT3/ITD patients.30, 31 We therefore examined whether WWP1 inactivation causes autophagic degradation of FLT3 in FLT3-ITD MV4-11 AML cells. We found that FLT3-ITD was indeed downregulated in WWP1-depleted MV4-11 cells (Figure 5e), implying that WWP1 influences the differentiation of FLT3-ITD AML cells by modulating the stability of the oncoprotein.
ATRA negatively controls WWP1 expression in APL cells
Pharmacological doses of ATRA induce myeloid differentiation of APL blasts, leading to disease remission in patients.32 As both ATRA and loss of WWP1 promote PML-RARα destabilization and granulocyte maturation of APL blasts, we sought to uncover potential mechanisms of cross-regulation. Treatment of NB4 cells with ATRA reduced WWP1 protein levels in a dose-dependent manner (Figure 5f), without significantly affecting WWP1 transcription (Supplementary Figure S6h). Of note, proteasomal inhibition was able to rescue ATRA-induced WWP1 downregulation (Figure 5g), thus demonstrating that ATRA interferes with WWP1 protein stability.
Coherently, exposure of NB4 cells to ATRA did not further destabilize PML-RARα and only moderately increased the expression of RARα target genes in WWP1-depleted cells (Supplementary Figures S6g–k). Therefore, ATRA treatment and WWP1 depletion display similar effects, but do not synergize in regulating PML-RARα stability and activity. These findings indicate that ATRA, partially, acts by promoting WWP1 disposal. The effect of ATRA on WWP1 deregulation seemed to be specific for APL cells, as other AML cell lines, which are partially or completely resistant to the differentiating effects of ATRA, did not modulate WWP1 in response to the retinoid (Supplementary Figure S6l).
Discussion
We show here that WWP1 is overexpressed in primary AMLs and in AML cell lines, as compared with normal haematopoietic cells. WWP1 downregulation negatively affects proliferation of AML blasts. Coherently, we found that WWP1 inactivation delays leukaemia progression in vivo.
Mechanistically, WWP1 inactivation in AML cells rapidly induces G0/G1 arrest, which is preceded by posttranslational stabilization of p27Kip (Figure 5h). We also found that WWP1 has a novel role in autophagy of AML cells (Figure 5h).
WWP1 is predominantly localized in cell membranes including the plasma membrane, endosomes and Golgi apparatus,33 which represent the main nucleation sites for the autophagosome.34 WWP1 subcellular localization is consistent with our observations that only the autophagosome-formation step appeared to be influenced by WWP1 depletion. Therefore, WWP1 may control autophagosome building by interfering with the degradation and/or function of proteins involved in the nucleation step. The molecular mechanisms through which WWP1 modulates autophagy remain to be elucidated.
Induction of autophagy is detectable shortly after inactivation of WWP1 and is followed by myeloid maturation of AML cells expressing oncoproteins, such as PML-RARα and FLT3/ITD, which are subjected to autophagy-dependent disposal (Figure 5h). Furthermore, the early kinetics of CD11b upregulation in these cell lines argues against the possibility that differentiation is the result of the G0/G1 cell cycle arrest. In addition, concomitantly to inducing cell cycle arrest, WWP1 loss diminished cell viability of leukaemic cells. The observation that only a portion of the AML cell lines underwent apoptosis opens different possible scenarios (Figure 5h). Although it is well established that WWP1 exerts an anti-apoptotic activity in epithelial cancer cells, relevant substrates mediating this effect are still largely unknown. It might be possible that these apoptotic regulators or effectors are mutated (for example, p53) or are not indispensable for most of AML cell lines to undergo apoptosis.
On the other hand, autophagic cell death may account for decreased cell viability observed in WWP1-depleted leukaemic cells. Cells facing excessive autophagy can indeed undergo cell death with no detectable caspase activation or other markers of apoptosis.24, 25, 26 However, the autophagic degradation of specific targets or the interaction of the autophagic machinery with some apoptotic regulators could promote apoptosis in other AML cell lines. Differently, apoptosis could be triggered in response to autophagic degradation of oncoproteins (Figure 5h). Disposal of FLT3/ITD through autophagy indeed leads to apoptosis.19
Given that E3s provide substrate specificity to the ubiquitination reaction, they represent attractive targets for developing therapeutic molecules that would increase the stability of tumour-suppressive proteins.35, 36 Here we demonstrated that, Heclin, a specific inhibitor of the HECT E3s, effectively reduced the growth potential of AML cells, thus providing a strong rationale for in vivo testing HECT of inhibitors against AML.
Our findings are clinically relevant for APL patients, because ATRA-based differentiation therapy, in part, drives the degradation of PML-RARα through autophagy induction.14, 15, 16, 17, 18 The observation that both ATRA and WWP1 loss promote myeloid maturation of leukaemic cells through PML-RARα destabilization could be of relevant clinical significance for those APL patients, who are resistant to initial ATRA therapy or develop resistance to ATRA monotherapy. In addition, beside chronic toxicity of As2O3, there still exist patients for whom As2O3 is not effective. Therefore, enhancement of autophagy by interfering with WWP1 activity alone or in combination with As2O3 may provide an alternative mean of overcoming treatment resistance in APL.
In AML patients bearing FLT3/ITD mutations, aberrant signalling from the mutant FLT3 receptor contributes to block granulocytic differentiation. FLT3 inhibition by tyrosine kinase inhibitor therapy (for example, quizartinib) induces in vivo terminal differentiation of FLT3/ITD AML blasts.30, 31 However, relapse often occurs mainly upon acquisition of secondary FLT3 kinase domain mutations that cause tyrosine kinase inhibitor resistance. Hence, alternative therapeutic strategies aimed to regulate FLT3-ITD levels are currently being investigated.19, 37 Proteasome inhibition has been shown to regulate the stability of FLT3-ITD through induction of autophagy.19 Similarly, we found that WWP1 inactivation causes autophagy-dependent FLT3-ITD downregulation, followed by myeloid maturation of leukaemic cells. Pharmacological inhibition of WWP1 might therefore represent an alternative therapeutic opportunity for FLT3-ITD inactivation to overcome tyrosine kinase inhibitor resistance.
More generally, WWP1 inhibition would have a relevant impact on other clinical subtypes, as resistance to chemotherapy or targeting drugs remains a challenging clinical task for AML. We indeed found that WWP1 depletion inhibited growth and reduced survival of different primary samples and cell lines representative of all AML subtypes. Patients with refractory or relapsed AML would indeed benefit from the anti-proliferative and cytotoxic effects of WWP1 inhibition.
Collectively, our findings support a role for WWP1 deregulation in sustaining leukaemia growth by promoting cell cycle entry and survival of AML blasts. In addition, WWP1 overexpression would contribute to the stabilization of leukaemogenic proteins targeted by autophagy. The in vivo anti-leukaemic effect of WWP1 inactivation is expected to be more pronounced in these AML subtypes, in which it promotes the release of the differentiation block of leukaemic blasts. This assumption is supported by the observation that silencing of WWP1 has a higher growth inhibiting effect on NB4 and MV4.11 cells.
Altogether, our data identify WWP1 as a potential therapeutic target in human AML.
References
Bernassola F, Karin M, Ciechanover A, Melino G . The HECT family of E3 ubiquitin ligases: multiple players in cancer development. Cancer Cell 2008; 14: 10–21.
Chen C, Sun X, Guo P, Dong XY, Sethi P, Zhou W et al. Ubiquitin E3 ligase WWP1 as an oncogenic factor in human prostate cancer. Oncogene 2007; 26: 2386–2394.
Cheng Q, Cao X, Yuan F, Li G, Tong T . Knockdown of WWP1 inhibits growth and induces apoptosis in hepatoma carcinoma cells through the activation of caspase3 and p53. Biochem Biophys Res Commun 2014; 448: 248–254.
Lin J-H, Hsieh S-C, Chen J-N, Tsai M-H, Chang C-C . WWP1 gene is a potential molecular target of human oral cancer. Oral Surg Oral Med Oral Pathol Oral Radiol 2013; 116: 221–231.
Zhang L, Wu Z, Ma Z, Liu H, Wu Y, Zhang Q . WWP1 as a potential tumor oncogene regulates PTEN-Akt signaling pathway in human gastric carcinoma. Tumour Biol 2015; 36: 787–798.
Chen C, Zhou Z, Ross JS, Zhou W, Dong J-T . The amplified WWP1 gene is a potential molecular target in breast cancer. Int J Cancer 2007; 121: 80–87.
Cao X, Xue L, Han L, Ma L, Chen T, Tong T . WW domain-containing E3 ubiquitin protein ligase 1 (WWP1) delays cellular senescence by promoting p27(Kip1) degradation in human diploid fibroblasts. J Biol Chem 2011; 286: 33447–33456.
Laine A, Ronai Z . Regulation of p53 localization and transcription by the HECT domain E3 ligase WWP1. Oncogene 2007; 26: 1477–1483.
Morén A, Imamura T, Miyazono K, Heldin C-H, Moustakas A . Degradation of the tumor suppressor Smad4 by WW and HECT domain ubiquitin ligases. J Biol Chem 2005; 280: 22115–22123.
Peschiaroli A, Scialpi F, Bernassola F, El Sherbini ES, Melino G . The E3 ubiquitin ligase WWP1 regulates ΔNp63-dependent transcription through Lys63 linkages. Biochem Biophys Res Commun 2010; 402: 425–430.
Gilliland DG . Hematologic malignancies. Curr Opin Hematol 2001; 8: 189–191.
Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 2013; 368: 2059–2074.
Shen Y, Zhu Y-M, Fan X, Shi JY, Wang QR, Yan XJ et al. Gene mutation patterns and their prognostic impact in a cohort of 1185 patients with acute myeloid leukemia. Blood 2011; 118: 5593–5603.
Isakson P, Bjørås M, Bøe SO, Simonsen A . Autophagy contributes to therapy-induced degradation of the PML/RARA oncoprotein. Blood 2010; 116: 2324–2331.
Wang Z, Cao L, Kang R, Yang M, Liu L, Zhao Y et al. Autophagy regulates myeloid cell differentiation by p62/SQSTM1-mediated degradation of PML-RARα oncoprotein. Autophagy 2011; 7: 401–411.
Goussetis DJ, Altman JK, Glaser H, McNeer JL, Tallman MS, Platanias LC . Autophagy is a critical mechanism for the induction of the antileukemic effects of arsenic trioxide. J Biol Chem 2010; 285: 29989–29997.
Brigger D, Proikas-Cezanne T, Tschan MP . WIPI-dependent autophagy during neutrophil differentiation of NB4 acute promyelocytic leukemia cells. Cell Death Dis 2014; 5: e1315.
Orfali N, O'Donovan TR, Nyhan MJ, Britschgi A, Tschan MP, Cahill MR et al. Induction of autophagy is a key component of all- trans -retinoic acid-induced differentiation in leukemia cells and a potential target for pharmacologic modulation. Exp Hematol 2015; 43: 781–793.
Larrue C, Saland E, Boutzen H, Vergez F, David M, Joffre C et al. Proteasome inhibitors induce FLT3-ITD degradation through autophagy in AML cells. Blood 2016; 127: 882–892.
Tormo D, Checińska A, Alonso-Curbelo D, Pérez-Guijarro E, Cañón E, Riveiro-Falkenbach E et al. Targeted activation of innate immunity for therapeutic induction of autophagy and apoptosis in melanoma cells. Cancer Cell 2009; 16: 103–114.
Workman P, Aboagye EO, Balkwill F, Balmain A, Bruder G, Chaplin DJ et al. Guidelines for the welfare and use of animals in cancer research. Br J Cancer 2010; 102: 1555–1577.
Mund T, Lewis MJ, Maslen S, Pelham HR . Peptide and small molecule inhibitors of HECT-type ubiquitin ligases. Proc Natl Acad Sci USA 2014; 111: 16736–16741.
Zhou Z, Liu R, Chen C . The WWP1 ubiquitin E3 ligase increases TRAIL resistance in breast cancer. Int J Cancer 2012; 130: 1504–1510.
Høyer-Hansen M, Bastholm L, Mathiasen IS, Elling F, Jäättelä M . Vitamin D analog EB1089 triggers dramatic lysosomal changes and Beclin 1-mediated autophagic cell death. Cell Death Differ 2005; 12: 1297–1309.
Qian W, Liu J, Jin J, Ni W, Xu W . Arsenic trioxide induces not only apoptosis but also autophagic cell death in leukemia cell lines via up-regulation of Beclin-1. Leuk Res 2007; 31: 329–339.
Tait SWG, Ichim G, Green DR . Die another way-non-apoptotic mechanisms of cell death. J Cell Sci 2014; 127: 2135–2144.
Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition. Autophagy 2016; 12: 1–222.
Grignani F, Ferrucci PF, Testa U, Talamo G, Fagioli M, Alcalay M et al. The acute promyelocytic leukemia-specific PML-RAR alpha fusion protein inhibits differentiation and promotes survival of myeloid precursor cells. Cell 1993; 74: 423–431.
Fanelli M, Minucci S, Gelmetti V, Nervi C, Gambacorti-Passerini C, Pelicci PG . Constitutive degradation of PML/RARalpha through the proteasome pathway mediates retinoic acid resistance. Blood 1999; 93: 1477–1481.
Zheng R, Friedman AD, Small D . Targeted inhibition of FLT3 overcomes the block to myeloid differentiation in 32Dcl3 cells caused by expression of FLT3/ITD mutations. Blood 2002; 100: 4154–4161.
Sexauer A, Perl A, Yang X, Borowitz M, Gocke C, Rajkhowa T et al. Terminal myeloid differentiation in vivo is induced by FLT3 inhibition in FLT3/ITD AML. Blood 2012; 120: 4205–4214.
Degos L, Dombret H, Chomienne C, Daniel MT, Micléa JM, Chastang C et al. All-trans-retinoic acid as a differentiating agent in the treatment of acute promyelocytic leukemia. Blood 1995; 85: 2643–2653.
Chen C, Zhou Z, Liu R, Li Y, Azmi PB, Seth AK . The WW domain containing E3 ubiquitin protein ligase 1 upregulates ErbB2 and EGFR through RING finger protein 11. Oncogene 2008; 27: 6845–6855.
Reggiori F, Tooze SA . Autophagy regulation through Atg9 traffic. J Cell Biol 2012; 198: 151–153.
Chène P . Inhibiting the p53-MDM2 interaction: an important target for cancer therapy. Nat. Rev. Cancer 2003; 3: 102–109.
Nalepa G, Rolfe M, Harper JW . Drug discovery in the ubiquitin-proteasome system. Nat. Rev. Drug Discov 2006; 5: 596–613.
Blum W, Schwind S, Tarighat SS, Geyer S, Eisfeld AK, Whitman S et al. Clinical and pharmacodynamic activity of bortezomib and decitabine in acute myeloid leukemia. Blood 2012; 119: 6025–6031.
Acknowledgements
We thank Dr Angelo Peschiaroli for helpful discussion and critical review of the manuscript, and Laura Tizzoni and Valentina Dallolio for qPCR assays. We are grateful to Marisol Soengas (CNIO, Madrid, Spain) for sharing the Cherry-GFP-LC3 lentiviral construct. This work was supported by ‘Uncovering Excellence, 2014 to FB, by ERC advanced grant 2013 Project Number 341131 to PGP and by AIRC 2014 IG15653, AIRC 5xmille MCO9979 and Fondazione Roma malattie Non trasmissibili Cronico-Degenerative (NCD) to GM.
Author contributions
AGS, CR, AC, EM, UAC, AD, SL and MD performed research. AB, PM, GS, GM, F Bertolini and FLC contributed new reagents/analytic tools. LG and GEMM analysed the bioinformatics data. GM, PGP and F Bernassola conceived, designed and supervised the study, and wrote the paper. The paper was reviewed and approved by all the authors
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Sanarico, A., Ronchini, C., Croce, A. et al. The E3 ubiquitin ligase WWP1 sustains the growth of acute myeloid leukaemia. Leukemia 32, 911–919 (2018). https://doi.org/10.1038/leu.2017.342
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2017.342
This article is cited by
-
The role of NEDD4 related HECT-type E3 ubiquitin ligases in defective autophagy in cancer cells: molecular mechanisms and therapeutic perspectives
Molecular Medicine (2023)
-
WWP1 E3 ligase at the crossroads of health and disease
Cell Death & Disease (2023)
-
The roles of ubiquitination in AML
Annals of Hematology (2023)
-
The dual role of autophagy in acute myeloid leukemia
Journal of Hematology & Oncology (2022)
-
The emerging role of WWP1 in cancer development and progression
Cell Death Discovery (2021)