Abstract
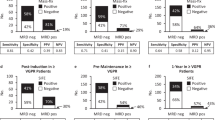
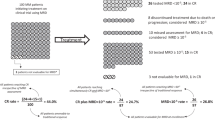
Persistence of minimal residual disease (MRD) after treatment for myeloma predicts inferior outcomes, but within MRD-positive patients there is great heterogeneity with both early and very late relapses. Among different MRD techniques, flow cytometry provides additional information about antigen expression on tumor cells, which could potentially contribute to stratify MRD-positive patients. We investigated the prognostic value of those antigens required to monitor MRD in 1265 newly diagnosed patients enrolled in the GEM2000, GEM2005MENOS65, GEM2005MAS65 and GEM2010MAS65 protocols. Overall, CD19pos, CD27neg, CD38lo, CD45pos, CD81pos, CD117neg and CD138lo expression predicted inferior outcomes. Through principal component analysis, we found that simultaneous CD38lowCD81posCD117neg expression emerged as the most powerful combination with independent prognostic value for progression-free survival (HR:1.69; P=0.002). This unique phenotypic profile retained prognostic value among MRD-positive patients. We then used next-generation flow to determine antigen stability throughout the course of the disease, and found that the expression of antigens required to monitor MRD is mostly stable from diagnosis to MRD stages, except for CD81 whose expression progressively increased from baseline to chemoresistant tumor cells (14 vs 28%). Altogether, we showed that the phenotypic profile of tumor cells provides additional prognostic information, and could be used to further predict risk of relapse among MRD-positive patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
San-Miguel JF, Hungria VT, Yoon SS, Beksac M, Dimopoulos MA, Elghandour A et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol 2014; 15: 1195–1206.
San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med [Internet] 2008; 359: 906–917.
Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Spicka I, Oriol A et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med 2015; 372: 142–152.
San Miguel J, Weisel K, Moreau P, Lacy M, Song K, Delforge M et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol 2013; 14: 1055–1066.
Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med 2015; 373: 621–631.
Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 2016; 375: 1319–1331.
Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med 2016; 375: 754–766.
Gormley NJ, Turley DM, Dickey JS, Farrell AT, Reaman GH, Stafford E et al. Regulatory perspective on minimal residual disease flow cytometry testing in multiple myeloma. Cytom B, Clin Cytom 2016; 90: 73–80.
Gormley NJ, Farrell AT, Pazdur R . Minimal residual disease as a potential surrogate end point-lingering questions. JAMA Oncol 2017; 3: 18–20.
Lahuerta J-J, Paiva B, Vidriales M-B, Cordón L, Cedena M-T, Puig N et al. Depth of response in multiple myeloma: a pooled analysis of three PETHEMA/GEM clinical trials. J Clin Oncol 2017; 35: 2900–2910, JCO2016692517.
Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 2016; 17: e328–e346.
Martinez-Lopez J, Lahuerta JJ, Pepin F, Gonzalez M, Barrio S, Ayala R et al. Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood [Internet] 2014; 123: 3073–3079.
Flores-Montero J, Flores LS, Paiva B, Puig N, Garcia-Sanchez O, Bottcher S et al. Next generation flow (NGF) for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia 2017; 31: 2094–2103.
Paiva B, Gutierrez NC, Rosinol L, Vidriales MB, Montalban MA, Martinez-Lopez J et al. High-risk cytogenetics and persistent minimal residual disease by multiparameter flow cytometry predict unsustained complete response after autologous stem cell transplantation in multiple myeloma. Blood [Internet] 2012; 119: 687–691.
Paiva B, Cedena MT, Puig N, Arana P, Vidriales MB, Cordon L et al. Minimal residual disease monitoring and immune profiling in multiple myeloma in elderly patients. Blood 2016; 127: 3165–3174.
Paiva B, Corchete LA, Vidriales MB, Puig N, Maiso P, Rodriguez I et al. Phenotypic and genomic analysis of multiple myeloma minimal residual disease tumor cells: a new model to understand chemoresistance. Blood 2016; 127: 1896–1906.
Paino T, Paiva B, Sayagues JM, Mota I, Carvalheiro T, Corchete LA et al. Phenotypic identification of subclones in multiple myeloma with different chemoresistant, cytogenetic and clonogenic potential. Leukemia 2015; 29: 1186–1194.
Paiva B, Puig N, Cedena MT, de Jong BG, Ruiz Y, Rapado I et al. Differentiation stage of myeloma plasma cells: biological and clinical significance. Leukemia 2017; 31: 382–392.
Gonsalves WI, Timm MM, Rajkumar SV, Morice WG, Dispenzieri A, Buadi FK et al. The prognostic significance of CD45 expression by clonal bone marrow plasma cells in patients with newly diagnosed multiple myeloma. Leuk Res 2016; 44: 32–39.
Paiva B, Gutierrez NC, Chen X, Vidriales MB, Montalban MA, Rosinol L et al. Clinical significance of CD81 expression by clonal plasma cells in high-risk smoldering and symptomatic multiple myeloma patients. Leukemia 2012; 26: 1862–1869.
Lahuerta JJ, Mateos MV, Martinez-Lopez J, Rosinol L, Sureda A, de la Rubia J et al. Influence of pre- and post-transplantation responses on outcome of patients with multiple myeloma: sequential improvement of response and achievement of complete response are associated with longer survival. J Clin Oncol [Internet] 2008; 26: 5775–5782, Available from.
Rosinol L, Oriol A, Teruel AI, Hernandez D, Lopez-Jimenez J, de la Rubia J et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood [Internet] 2012; 120: 1589–1596.
Mateos MV, Oriol A, Martinez-Lopez J, Teruel AI, Lopez de la Guia A, Lopez J et al. GEM2005 trial update comparing VMP/VTP as induction in elderly multiple myeloma patients: do we still need alkylators? Blood 2014; 124: 1887–1893.
Mateos MV, Martinez-Lopez J, Hernandez MT, Ocio EM, Rosinol L, Martinez R et al. Sequential vs alternating administration of VMP and Rd in elderly patients with newly diagnosed MM. Blood 2016; 127: 420–425.
Paiva B, Vidriales MB, Mateo G, Perez JJ, Montalban MA, Sureda A et al. The persistence of immunophenotypically normal residual bone marrow plasma cells at diagnosis identifies a good prognostic subgroup of symptomatic multiple myeloma patients. Blood 2009; 114: 4369–4372.
van Dongen JJ, Lhermitte L, Bottcher S, Almeida J, van der Velden VH, Flores-Montero J et al. EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia 2012; 26: 1908–1975.
Harousseau J-L, Avet-Loiseau H . Minimal residual disease negativity is a new end point of myeloma therapy. J Clin Oncol 2017; 35: 2863–2865.
Zeijlemaker W, Gratama JW, Schuurhuis GJ . Tumor heterogeneity makes AML a ‘moving target’ for detection of residual disease. Cytom B, Clin Cytom 2014; 86: 3–14.
Avet-Loiseau H, Corre J, Lauwers-Cances V, Chretien M-L, Robillard N, Leleu X et al. Evaluation of minimal residual disease (MRD) by next generation sequencing (NGS) is highly predictive of progression free survival in the IFM/DFCI 2009 trial. Blood [Internet] 2015; 126: 191, Available from http://www.bloodjournal.org/content/126/23/191.abstract.
de Tute RM, Rawstron AC, Gregory WM, Child JA, Davies FE, Bell SE et al. Minimal residual disease following autologous stem cell transplant in myeloma: impact on outcome is independent of induction regimen. Haematologica 2016; 101: e69–e71.
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med 2017; 376: 1311–1320.
Avet-Loiseau H, Casneuf T, Chiu C, Laubach JP, Lee J-J, Moreau P et al. Evaluation of minimal residual disease (MRD) in relapsed/refractory multiple myeloma (RRMM) patients treated with daratumumab in combination with lenalidomide plus dexamethasone or bortezomib plus dexamethasone. Blood [Internet] 2016; 128: 246 LP–246246, Available from http://www.bloodjournal.org/content/128/22/246.abstract.
Rawstron AC, Child JA, de Tute RM, Davies FE, Gregory WM, Bell SE et al. Minimal residual disease assessed by multiparameter flow cytometry in multiple myeloma: impact on outcome in the Medical Research Council Myeloma IX Study. J Clin Oncol [Internet] 2013; 31: 2540–2547.
Schmidt-Hieber M, Perez-Andres M, Paiva B, Flores-Montero J, Perez JJ, Gutierrez NC et al. CD117 expression in gammopathies is associated with an altered maturation of the myeloid and lymphoid hematopoietic cell compartments and favorable disease features. Haematologica 2011; 96: 328–332.
Nijhof IS, Groen RWJ, Lokhorst HM, van Kessel B, Bloem AC, van Velzen J et al. Upregulation of CD38 expression on multiple myeloma cells by all-trans retinoic acid improves the efficacy of daratumumab. Leukemia 2015; 29: 2039–2049.
Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E et al. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev 2008; 88: 841–886.
Deaglio S, Aydin S, Vaisitti T, Bergui L, Malavasi F . CD38 at the junction between prognostic marker and therapeutic target. Trends Mol Med 2008; 14: 210–218.
Deaglio S, Morra M, Mallone R, Ausiello CM, Prager E, Garbarino G et al. Human CD38 (ADP-ribosyl cyclase) is a counter-receptor of CD31, an Ig superfamily member. J Immunol 1998; 160: 395–402.
Paiva B, Paino T, Sayagues JM, Garayoa M, San-Segundo L, Martin M et al. Detailed characterization of multiple myeloma circulating tumor cells shows unique phenotypic, cytogenetic, functional, and circadian distribution profile. Blood 2013; 122: 3591–3598.
Chaidos A, Barnes CP, Cowan G, May PC, Melo V, Hatjiharissi E et al. Clinical drug resistance linked to interconvertible phenotypic and functional states of tumor-propagating cells in multiple myeloma. Blood 2013; 121: 318–328.
Perez-Andres M, Almeida J, Martin-Ayuso M, De Las Heras N, Moro MJ, Martin-Nunez G et al. Soluble and membrane levels of molecules involved in the interaction between clonal plasma cells and the immunological microenvironment in multiple myeloma and their association with the characteristics of the disease. Int J Cancer 2009; 124: 367–375.
Nijhof IS, Casneuf T, van Velzen J, van Kessel B, Axel AE, Syed K et al. CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood 2016; 128: 959–970.
Acknowledgements
We would like to acknowledge Arturo Touchard for outstanding data management and all the investigators of GEM/PETHEMA. This study was supported by the Centro de Investigación Biomédica en Red—Área de Oncología—del Instituto de Salud Carlos III (CIBERONC; CB16/12/00369; CB16/12/00400; CB16/12/00233; CB16/12/00284), formerly named as Cooperative Research Thematic Network (Grants No. RD12/0036/0058, RD12/0036/0048, RD12/0036/0046 and RD12/0036/0061) of the Red de Cancer (Cancer Network of Excellence); Instituto de Salud Carlos III/Subdirección General de Investigación Sanitaria; funded in part by the European Regional Development Fund (FIS No. 98/1239, 00/10160, 01/0089, 02/0089, 02/0905, G03/136, PI051284, PI06033906/1354, PS09/01897/01370, PI12/01761, PI12/ 02311, PI13/01469, PI14/01867, G03/136); Sara Borrell (No. CD13/00340); Asociación Española Contra el Cáncer (GCB120981SAN) and the Becas Leonardo a Investigadores y Creadores Culturales 2017, Fundación BBVA. This study was also supported internationally by the Black Swan Research Initiative of the International Myeloma Foundation, the Qatar National Research Fund (QNRF) Award No. 7-916-3-237, the AACR-Millennium Fellowship in Multiple Myeloma Research (15-40-38-PAIV), the Leukemia Research Foundation, and the European Research Council (ERC) 2015 Starting Grant (MYELOMANEXT).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Arana, P., Paiva, B., Cedena, MT. et al. Prognostic value of antigen expression in multiple myeloma: a PETHEMA/GEM study on 1265 patients enrolled in four consecutive clinical trials. Leukemia 32, 971–978 (2018). https://doi.org/10.1038/leu.2017.320
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2017.320
This article is cited by
-
An inflammatory response-related gene signature can predict the prognosis and impact the immune infiltration of multiple myeloma
Clinical and Experimental Medicine (2024)
-
Immunophenotypic profile defines cytogenetic stability and unveils distinct prognoses in patients with newly-diagnosed multiple myeloma (NDMM)
Annals of Hematology (2024)
-
Mass Cytometry reveals unique phenotypic patterns associated with subclonal diversity and outcomes in multiple myeloma
Blood Cancer Journal (2023)
-
CD34+ myeloma cells with self-renewal activities are therapy-resistant and persist as MRD in cell cycle quiescence
International Journal of Hematology (2022)
-
The surfaceome of multiple myeloma cells suggests potential immunotherapeutic strategies and protein markers of drug resistance
Nature Communications (2022)