Abstract
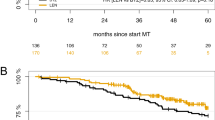
In newly diagnosed myeloma patients, upfront autologous transplant (ASCT) prolongs progression-free survival 1 (PFS1) compared with chemotherapy plus lenalidomide (CC+R). Salvage ASCT at first relapse may still effectively rescue patients who did not receive upfront ASCT. To evaluate the long-term benefit of upfront ASCT vs CC+R and the impact of salvage ASCT in patients who received upfront CC+R, we conducted a pooled analysis of 2 phase III trials (RV-MM-209 and EMN-441). Primary endpoints were PFS1, progression-free survival 2 (PFS2), overall survival (OS). A total of 268 patients were randomized to 2 courses of melphalan 200 mg/m2 and ASCT (MEL200-ASCT) and 261 to CC+R. Median follow-up was 46 months. MEL200-ASCT significantly improved PFS1 (median: 42 vs 24 months, HR 0.53; P<0.001), PFS2 (4 years: 71 vs 54%, HR 0.53, P<0.001) and OS (4 years: 84 vs 70%, HR 0.51, P<0.001) compared with CC+R. The advantage was noticed in good and bad prognosis patients. Only 53% of patients relapsing from CC+R received ASCT at first relapse. Upfront ASCT significantly reduced the risk of death (HR 0.51; P=0.007) in comparison with salvage ASCT. In conclusion, these data confirm the role of upfront ASCT as the standard approach for all young myeloma patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF et al SEER cancer statistics review, 1975–2010. Bethesda, MD, USA: National Cancer Institute, based on November 2012 SEER data submission, posted to the SEER Web site, 2013. Available at http://seer.cancer.gov/csr/1975_2010/.
Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008; 111: 2516–2520.
Ludwig H, Sonneveld P, Davies F, Bladé J, Boccadoro M, Cavo M et al. European perspective on multiple myeloma treatment strategies in 2014. Oncologist 2014; 19: 829–844.
Moreau P, Attal M, Facon T . Frontline therapy of multiple myeloma. Blood 2015; 125: 3076–3084.
Fayers PM, Palumbo A, Hulin C, Waage A, Wijermans P, Beksaç M et al. Thalidomide for previously untreated elderly patients with multiple myeloma: meta-analysis of 1685 individual patient data from 6 randomized clinical trials. Blood 2011; 118: 1239–1247.
San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med 2008; 359: 906–917.
Benboubker L, Dimopoulos MA, Dispenzieri A, Catalano J, Belch AR, Cavo M et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med 2014; 371: 906–917.
Palumbo A, Cavallo F, Gay F, Di Raimondo F, Ben Yehuda D, Petrucci MT et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med 2014; 371: 895–905.
Gay F, Oliva S, Petrucci MT, Conticello C, Catalano L, Corradini P et al. Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. Lancet Oncol 2015; 16: 1617–1629.
Attal M, Lauwers-Cances V, Hulin C, Facon T, Caillot D, Escoffre M et al. Autologous transplantation for multiple myeloma in the era of new drugs: a Phase III Study of the Intergroupe Francophone Du Myelome (IFM/DFCI 2009 Trial) [abstract]. Blood 2015; 126: 731 (abstr 391).
Cavo M, Palumbo A, Zweegman S, Dimopoulos MA, Hajek R, Pantani L et al. Upfront autologous stem cell transplantation (ASCT) versus novel agent-based therapy for multiple myeloma (MM): A randomized phase 3 study of the European Myeloma Network (EMN02/HO95 MM trial). J Clin Oncol 2016, 34 (abstr 8000).
Koreth J, Cutler CS, Djulbegovic B, Behl R, Schlossman RL, Munshi NC et al. High-dose therapy with single autologous transplantation versus chemotherapy for newly diagnosed multiple myeloma: A systematic review and meta-analysis of randomized controlled trials. Biol Blood Marrow Transplant 2007; 13: 183–196.
Fermand JP, Ravaud P, Chevret S, Divine M, Leblond V, Belanger C et al. High-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood 1998; 92: 3131–3136.
Palumbo A, Bringhen S, Larocca A, Rossi D, Di Raimondo F, Magarotto V et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: updated follow-up and improved survival. J Clin Oncol 2014; 32: 634–640.
Rajkumar SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol 2010; 11: 29–37.
Kumar SK, Lacy MQ, Dispenzieri A, Buadi FK, Hayman SR, Dingli D et al. Early versus delayed autologous transplantation after immunomodulatory agents-based induction therapy in patients with newly diagnosed multiple myeloma. Cancer 2012; 118: 1585–1592.
Dunavin NC, Wei L, Elder P, Phillips GS, Benson DM Jr, Hofmeister CC et al. Early versus delayed autologous stem cell transplant in patients receiving novel therapies for multiple myeloma. Leuk Lymphoma 2013; 54: 1658–1664.
Biran N, Jacobus S, Vesole DH, Callander NS, Fonseca R, Williams ME et al. Outcome with lenalidomide plus dexamethasone followed by early autologous stem cell transplantation in patients with newly diagnosed multiple myeloma on the ECOG-ACRIN E4A03 randomized clinical trial: long-term follow-up. Blood Cancer J 2016; 6: e466.
Greipp PR, San MJ, Durie BG, Crowley JJ, Barlogie B, Bladé J et al. International staging system for multiple myeloma. J Clin Oncol 2005; 23: 3412–3420.
Rajkumar SV, Harousseau J-L, Durie B, Anderson KC, Dimopoulos M, Kyle R et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood 2011; 117: 4691–4695.
Harrell FrankE Jr . Missing Data. Multiple Imputation. In: Regression Modeling Strategies With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. Springer, 2015; pp 53–56.
Facon T, Mary JY, Hulin C, Benboubker L, Attal M, Pegourie B et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet 2007; 370: 1209–1218.
Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med 1996; 335: 91–97.
Fermand JP, Katsahian S, Divine M, Leblond V, Dreyfus F, Macro M et al. High-dose therapy and autologous blood stem-cell transplantation compared with conventional treatment in myeloma patients aged 55 to 65 years: long-term results of a randomized control trial from the Group Myelome-Autogreffe. J Clin Oncol 2005; 23: 9227–9233.
Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med 2003; 348: 1875–1883.
Barlogie B, Kyle RA, Anderson KC, Greipp PR, Lazarus HM, Hurd DD et al. Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: final results of phase III US Intergroup Trial S9321. J Clin Oncol 2006; 24: 929–936.
Bladé J, Rosiñol L, Sureda A, Ribera JM, Díaz-Mediavilla J, García-Laraña J et al. High-dose therapy intensification compared with continued standard chemotherapy in multiple myeloma patients responding to the initial chemotherapy: long-term results from a prospective randomized trial from the Spanish cooperative group PETHEMA. Blood 2005; 106: 3755–3759.
Segeren CM, Sonneveld P, van der Holt B, Vellenga E, Croockewit AJ, Verhoef GE et al. Overall and event-free survival are not improved by the use of myeloablative therapy following intensified chemotherapy in previously untreated patients with multiple myeloma: a prospective randomized phase 3 study. Blood 2003; 101: 2144–2151.
Palumbo A, Bringhen S, Petrucci MT, Musto P, Rossini F, Nunzi M et al. Intermediate-dose melphalan improves survival of myeloma patients aged 50 to 70: results of a randomized controlled trial. Blood 2004; 104: 3052–3057.
Sonneveld P, Schmidt-Wolf IG, van der Holt B, El Jarari L, Bertsch U, Salwender H et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/ GMMG-HD4 trial. J Clin Oncol 2012; 30: 2946–2955.
Cavo M, Pantani L, Pezzi A, Petrucci MT, Petrucci MT, Patriarca F et al. Bortezomib-thalidomide-dexamethasone (VTD) is superior to bortezomib-cyclophosphamide-dexamethasone (VCD) as induction therapy prior to autologous stem cell transplantation in multiple myeloma. Leukemia 2015; 29: 2429–2431.
Cavo M, Pantani L, Petrucci MT, Patriarca F, Zamagni E, Donnarumma D et al. Bortezomib-thalidomide-dexamethasone is superior to thalidomide-dexamethasone as consolidation therapy after autologous hematopoietic stem cell transplantation in patients with newly diagnosed multiple myeloma. Blood 2012; 120: 9–19.
Harousseau JL, Attal M, Avet-Loiseau H, Marit G, Caillot D, Mohty M et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: Results of the IFM 2005-01 phase III trial. J Clin Oncol 2010; 28: 4621–4629.
Cavo M, Tacchetti P, Patriarca F, Petrucci MT, Pantani L, Galli M et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: A randomised phase 3 study. Lancet 2010; 376: 2075–2085.
Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M et al. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N Engl J Med 2016; 375: 754–766.
Dimopoulos MA, Oriol A, Nahi H, Miguel JS, Bahlis NJ, Rabin N et al. An Open-Label, Randomised, Phase 3 Study of Daratumumab, Lenalidomide, and Dexamethasone (Drd) versus lenalidomide and dexamethasone (Rd) in relapsed or refractory multiple myeloma (Rrmm): Pollux. Eur Hematol Assoc 2016; 101 (Suppl 1): (abstract LB2238).
Acknowledgements
We thank all the patients who participated in the source studies; the Australasian Leukaemia and Lymphoma Group (ALLG); the nurses Luisella D’Ambrosio and Tiziana De Lazzer, the data managers Marta Santoro and Federica Leotta, and the editorial assistant Giorgio Schirripa from Torino site. The RV-MM-PI-209 and the EMN-441 studies were sponsored by Fondazione Neoplasie Sangue Onlus and supported by Celgene. The funder of the source studies had no role in the present study, and no funding was received for this analysis.
Author contributions
FG, AP and MB designed the study; FG and AP collected and assembled the data; FG, AE, SS, MS, AP and MB analyzed and interpreted the data; FG wrote the first draft of the manuscript; all authors had access to the final data and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
FG has received honoraria from Amgen, BMS, Celgene and Takeda, and served on the advisory committee for Janssen, Mundipharma, and Takeda. SO has received honoraria from Takeda and Celgene. MTP has received honoraria from Celgene, Janssen-Cilag, Bristol-Myers Squibb, Amgen, Takeda, Mundipharma, Sanofi. PM has received honoraria from Celgene, Janssen, Novartis, Sanofi, Bristol-Myers Squibb, Takeda, Amgen. MO has received honoraria from Celgene. TC has received honoraria from Celgene, Janssen, Amgen, Bristol-Myers Squibb, and consultancy fees from Takeda. FP has received honoraria from MSD Italia, Celgene, and served on the advisory board of Janssen, Mundipharma, Amgen, Bristol-Meyers Squibb. AS has received honoraria from Celgene. RH has received consultancy fees from Celgene, Janssen, and honoraria from Amgen. AP has received consultancy fees, honoraria and research funding from Celgene, and is a Takeda employee. MB has received consultancy fees from Janssen, Sanofi, Onyx, Amgen, Celgene. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Gay, F., Oliva, S., Petrucci, M. et al. Autologous transplant vs oral chemotherapy and lenalidomide in newly diagnosed young myeloma patients: a pooled analysis. Leukemia 31, 1727–1734 (2017). https://doi.org/10.1038/leu.2016.381
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2016.381
This article is cited by
-
Melphalan 200 mg/m2 does not increase toxicity and improves survival in comparison to reduced doses of melphalan in multiple myeloma patients
Bone Marrow Transplantation (2021)
-
Clonal hematopoiesis is associated with adverse outcomes in multiple myeloma patients undergoing transplant
Nature Communications (2020)
-
Utilization of hematopoietic stem cell transplantation for the treatment of multiple myeloma: a Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus statement
Bone Marrow Transplantation (2019)
-
Impact of post-transplantation maintenance therapy on health-related quality of life in patients with multiple myeloma: data from the Connect® MM Registry
Annals of Hematology (2018)
-
A phase 1 trial of 90Y-Zevalin radioimmunotherapy with autologous stem cell transplant for multiple myeloma
Bone Marrow Transplantation (2017)