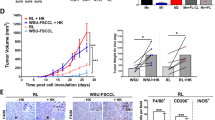
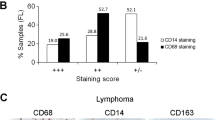
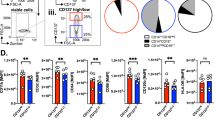
Abstract
The development and progression of chronic B-cell tumors depend on a complex microenvironmental network of cells that include monocyte-derived macrophages. In chronic lymphocytic leukemia (CLL) the survival of malignant cells is supported in vitro by nurse-like cells, which differentiate from CD14+ monocytes and have been identified as tumor-associated macrophages (TAMs). The role of the monocyte/macrophage lineage in CLL has been extensively studied in vitro, but only recently has been investigated in in vivo models. We here discuss how the cellular and molecular interactions that physiologically occur between B cells and macrophages can be subverted in chronic B lymphoid malignancies. Clinical approaches for the therapeutic targeting of TAMs are under evaluation. Promising strategies, along with a direct impact on the malignant cells, affect crucial pathways involved in the interaction of leukemic cells with TAMs. As an example, ibrutinib reduces CLL cell chemoattraction by inhibiting macrophage secretion of CXCL13. Lenalidomide and trabectedin prevent TAM recruitment mainly through CCL2 blockade. Most advanced strategies aim at depleting macrophages by targeting the CSF1/CSF1R pathway, which is fundamental for TAM survival. Of note, CSF1 transcripts are significantly more abundant in progressive CLL patients when compared with stable CLL and the frequency of CSF1R+ TAMs correlates with poor survival in hematological malignancies. The successful combination of CSF1R inhibition with currently available agents targeting malignant cells might represent the next therapeutic frontier in CLL. Conceivably these approaches may become applicable to numerous chronic B lymphoid malignancies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Burger JA, Ghia P, Rosenwald A, Caligaris-Cappio F . The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood 2009; 114: 3367–3375.
Ding W, Nowakowski GS, Knox TR, Boysen JC, Maas ML, Schwager SM et al. Bi-directional activation between mesenchymal stem cells and CLL B-cells: implication for CLL disease progression. Br J Haematol 2009; 147: 471–483.
Burger JA . Nurture versus nature: the microenvironment in chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program 2011; 2011: 96–103.
Burger JA, Gribben JG . The microenvironment in chronic lymphocytic leukemia (CLL) and other B cell malignancies: insight into disease biology and new targeted therapies. Semin Cancer Biol 2014; 24: 71–81.
Ten Hacken E, Burger JA . Microenvironment interactions and B-cell receptor signaling in Chronic lymphocytic leukemia: implications for disease pathogenesis and treatment. Biochim Biophys Acta 2016; 1863: 401–413.
Heinig K, Gatjen M, Grau M, Stache V, Anagnostopoulos I, Gerlach K et al. Access to follicular dendritic cells is a pivotal step in murine chronic lymphocytic leukemia B-cell activation and proliferation. Cancer Discov 2014; 4: 1448–1465.
Pedersen IM, Kitada S, Leoni LM, Zapata JM, Karras JG, Tsukada N et al. Protection of CLL B cells by a follicular dendritic cell line is dependent on induction of Mcl-1. Blood 2002; 100: 1795–1801.
Ramsay AG, Johnson AJ, Lee AM, Gorgun G, Le Dieu R, Blum W et al. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Invest 2008; 118: 2427–2437.
Ramsay AG, Clear AJ, Fatah R, Gribben JG . Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide: establishing a reversible immune evasion mechanism in human cancer. Blood 2012; 120: 1412–1421.
Patten PE, Buggins AG, Richards J, Wotherspoon A, Salisbury J, Mufti GJ et al. CD38 expression in chronic lymphocytic leukemia is regulated by the tumor microenvironment. Blood 2008; 111: 5173–5181.
Burger JA, Quiroga MP, Hartmann E, Burkle A, Wierda WG, Keating MJ et al. High-level expression of the T-cell chemokines CCL3 and CCL4 by chronic lymphocytic leukemia B cells in nurselike cell cocultures and after BCR stimulation. Blood 2009; 113: 3050–3058.
Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol 2014; 14: 571–578.
Varol C, Mildner A, Jung S . Macrophages: development and tissue specialization. Annu Rev Immunol 2015; 33: 643–675.
Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014; 41: 14–20.
De Palma M, Lewis CE . Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell 2013; 23: 277–286.
Noy R, Pollard JW . Tumor-associated macrophages: from mechanisms to therapy. Immunity 2014; 41: 49–61.
Ruffell B, Coussens LM . Macrophages and therapeutic resistance in cancer. Cancer Cell 2015; 27: 462–472.
Affara NI, Ruffell B, Medler TR, Gunderson AJ, Johansson M, Bornstein S et al. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell 2014; 25: 809–821.
Gabrilovich DI, Ostrand-Rosenberg S, Bronte V . Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012; 12: 253–268.
Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res 2010; 70: 5728–5739.
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A . Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002; 23: 549–555.
Pollard JW . Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 2004; 4: 71–78.
Ostuni R, Kratochvill F, Murray PJ, Natoli G . Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol 2015; 36: 229–239.
Chitu V, Stanley ER . Colony-stimulating factor-1 in immunity and inflammation. Curr Opin Immunol 2006; 18: 39–48.
Allavena P, Sica A, Solinas G, Porta C, Mantovani A . The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol 2008; 66: 1–9.
Solinas G, Germano G, Mantovani A, Allavena P . Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol 2009; 86: 1065–1073.
De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 2005; 8: 211–226.
Batista FD, Harwood NE . The who, how and where of antigen presentation to B cells. Nat Rev Immunol 2009; 9: 15–27.
DeKoter RP, Singh H . Regulation of B lymphocyte and macrophage development by graded expression of PU.1. Science 2000; 288: 1439–1441.
Kueh HY, Champhekhar A, Nutt SL, Elowitz MB, Rothenberg EV . Positive feedback between PU.1 and the cell cycle controls myeloid differentiation. Science 2013; 341: 670–673.
Jopling C, Boue S, Izpisua Belmonte JC . Dedifferentiation, transdifferentiation and reprogramming: three routes to regeneration. Nat Rev Mol Cell Biol 2011; 12: 79–89.
Montecino-Rodriguez E, Dorshkind K . Identification of B/macrophage progenitors in adult bone marrow. Semin Immunol 2002; 14: 371–376.
Hardy RR, Kincade PW, Dorshkind K . The protean nature of cells in the B lymphocyte lineage. Immunity 2007; 26: 703–714.
Cumano A, Paige CJ, Iscove NN, Brady G . Bipotential precursors of B cells and macrophages in murine fetal liver. Nature 1992; 356: 612–615.
Almeida SR, Aroeira LS, Frymuller E, Dias MA, Bogsan CS, Lopes JD et al. Mouse B-1 cell-derived mononuclear phagocyte, a novel cellular component of acute non-specific inflammatory exudate. Int Immunol 2001; 13: 1193–1201.
Borrello MA, Palis J, Phipps RP . The relationship of CD5+ B lymphocytes to macrophages: insights from normal biphenotypic B/macrophage cells. Int Rev Immunol 2001; 20: 137–155.
Craxton A, Magaletti D, Ryan EJ, Clark EA . Macrophage- and dendritic cell dependent regulation of human B-cell proliferation requires the TNF family ligand BAFF. Blood 2003; 101: 4464–4471.
Cerutti A, Cols M, Puga I . Activation of B cells by non-canonical helper signals. EMBO Rep 2012; 13: 798–810.
Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science 1999; 285: 260–263.
O'Garra A, Chang R, Go N, Hastings R, Haughton G, Howard M et al. (B-1) cells are the main source of B cell-derived interleukin 10. Eur J Immunol 1992; 22: 711–717.
Thies FG, Laurindo MF, Perez EC, Novaes e Brito RR, Mariano M, Popi AF . Cross talk between peritoneal macrophages and B-1 cells in vitro. PLoS One 2013; 8: e62805.
Popi AF, Lopes JD, Mariano M . Interleukin-10 secreted by B-1 cells modulates the phagocytic activity of murine macrophages in vitro. Immunology 2004; 113: 348–354.
Wong SC, Puaux AL, Chittezhath M, Shalova I, Kajiji TS, Wang X et al. Macrophage polarization to a unique phenotype driven by B cells. Eur J Immunol 2010; 40: 2296–2307.
Tacke F, Ginhoux F, Jakubzick C, van Rooijen N, Merad M, Randolph GJ . Immature monocytes acquire antigens from other cells in the bone marrow and present them to T cells after maturing in the periphery. J Exp Med 2006; 203: 583–597.
Varol C, Yona S, Jung S . Origins and tissue-context-dependent fates of blood monocytes. Immunol Cell Biol 2009; 87: 30–38.
Szakal AK, Holmes KL, Tew JG . Transport of immune complexes from the subcapsular sinus to lymph node follicles on the surface of nonphagocytic cells, including cells with dendritic morphology. J Immunol 1983; 131: 1714–1727.
Gray EE, Cyster JG . Lymph node macrophages. J Innate Immun 2012; 4: 424–436.
Gaya M, Castello A, Montaner B, Rogers N, Reis e Sousa C, Bruckbauer A et al. Host response. Inflammation-induced disruption of SCS macrophages impairs B cell responses to secondary infection. Science 2015; 347: 667–672.
Martinez-Pomares L, Gordon S . Antigen presentation the macrophage way. Cell 2007; 131: 641–643.
Phan TG, Grigorova I, Okada T, Cyster JG . Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat Immunol 2007; 8: 992–1000.
Martinez-Pomares L, Kosco-Vilbois M, Darley E, Tree P, Herren S, Bonnefoy JY et al. Fc chimeric protein containing the cysteine-rich domain of the murine mannose receptor binds to macrophages from splenic marginal zone and lymph node subcapsular sinus and to germinal centers. J Exp Med 1996; 184: 1927–1937.
Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 2007; 450: 110–114.
Carrasco YR, Batista FD . B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity 2007; 27: 160–171.
Phan TG, Green JA, Gray EE, Xu Y, Cyster JG . Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat Immunol 2009; 10: 786–793.
Moseman EA, Iannacone M, Bosurgi L, Tonti E, Chevrier N, Tumanov A et al. B cell maintenance of subcapsular sinus macrophages protects against a fatal viral infection independent of adaptive immunity. Immunity 2012; 36: 415–426.
Mohr E, Serre K, Manz RA, Cunningham AF, Khan M, Hardie DL et al. Dendritic cells and monocyte/macrophages that create the IL-6/APRIL-rich lymph node microenvironments where plasmablasts mature. J Immunol 2009; 182: 2113–2123.
You Y, Myers RC, Freeberg L, Foote J, Kearney JF, Justement LB et al. Marginal zone B cells regulate antigen capture by marginal zone macrophages. J Immunol 2011; 186: 2172–2181.
Veninga H, Borg EG, Vreeman K, Taylor PR, Kalay H, van Kooyk Y et al. Antigen targeting reveals splenic CD169+ macrophages as promoters of germinal center B-cell responses. Eur J Immunol 2015; 45: 747–757.
Nikbakht N, Shen S, Manser T . Cutting edge: macrophages are required for localization of antigen-activated B cells to the follicular perimeter and the subsequent germinal center response. J Immunol 2013; 190: 4923–4927.
Mueller CG, Cremer I, Paulet PE, Niida S, Maeda N, Lebeque S et al. Mannose receptor ligand-positive cells express the metalloprotease decysin in the B cell follicle. J Immunol 2001; 167: 5052–5060.
Groeneveld PH, Erich T, Kraal G . The differential effects of bacterial lipopolysaccharide (LPS) on splenic non-lymphoid cells demonstrated by monoclonal antibodies. Immunology 1986; 58: 285–290.
Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science 2001; 293: 2111–2114.
McGaha TL, Chen Y, Ravishankar B, van Rooijen N, Karlsson MC . Marginal zone macrophages suppress innate and adaptive immunity to apoptotic cells in the spleen. Blood 2011; 117: 5403–5412.
Dogusan Z, Montecino-Rodriguez E, Dorshkind K . Macrophages and stromal cells phagocytose apoptotic bone marrow-derived B lineage cells. J Immunol 2004; 172: 4717–4723.
Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T et al. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N Engl J Med 2010; 362: 875–885.
Kamper P, Bendix K, Hamilton-Dutoit S, Honore B, Nyengaard JR, d'Amore F . Tumor-infiltrating macrophages correlate with adverse prognosis and Epstein-Barr virus status in classical Hodgkin's lymphoma. Haematologica 2011; 96: 269–276.
Martin-Moreno AM, Roncador G, Maestre L, Mata E, Jimenez S, Martinez-Torrecuadrada JL et al. CSF1R protein expression in reactive lymphoid tissues and lymphoma: its relevance in classical Hodgkin lymphoma. PLoS One 2015; 10: e0125203.
Farinha P, Masoudi H, Skinnider BF, Shumansky K, Spinelli JJ, Gill K et al. Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL). Blood 2005; 106: 2169–2174.
Epron G, Ame-Thomas P, Le Priol J, Pangault C, Dulong J, Lamy T et al. Monocytes and T cells cooperate to favor normal and follicular lymphoma B-cell growth: role of IL-15 and CD40L signaling. Leukemia 2012; 26: 139–148.
Mitchell R, Bacher M, Bernhagen J, Pushkarskaya T, Seldin MF, Bucala R . Cloning and characterization of the gene for mouse macrophage migration inhibitory factor (MIF). J Immunol 1995; 154: 3863–3870.
Calandra T, Bernhagen J, Mitchell RA, Bucala R . The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med 1994; 179: 1895–1902.
Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T et al. MIF as a glucocorticoid-induced modulator of cytokine production. Nature 1995; 377: 68–71.
Talos F, Mena P, Fingerle-Rowson G, Moll U, Petrenko O . MIF loss impairs Myc-induced lymphomagenesis. Cell Death Differ 2005; 12: 1319–1328.
Asimakopoulos F, Kim J, Denu RA, Hope C, Jensen JL, Ollar SJ et al. Macrophages in multiple myeloma: emerging concepts and therapeutic implications. Leuk Lymphoma 2013; 54: 2112–2121.
Wada N, Zaki MA, Hori Y, Hashimoto K, Tsukaguchi M, Tatsumi Y et al. Tumour-associated macrophages in diffuse large B-cell lymphoma: a study of the Osaka Lymphoma Study Group. Histopathology 2012; 60: 313–319.
Nam SJ, Go H, Paik JH, Kim TM, Heo DS, Kim CW et al. An increase of M2 macrophages predicts poor prognosis in diffuse large B-cell lymphoma patients treated with Rituximab-CHOP. Leuk Lymphoma 2014; 55: 2466–2476.
Zheng Y, Cai Z, Wang S, Zhang X, Qian J, Hong S et al. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood 2009; 114: 3625–3628.
Kim J, Denu RA, Dollar BA, Escalante LE, Kuether JP, Callander NS et al. Macrophages and mesenchymal stromal cells support survival and proliferation of multiple myeloma cells. Br J Haematol 2012; 158: 336–346.
Zheng Y, Yang J, Qian J, Qiu P, Hanabuchi S, Lu Y et al. PSGL-1/selectin and ICAM-1/CD18 interactions are involved in macrophage-induced drug resistance in myeloma. Leukemia 2013; 27: 702–710.
Jaiswal S, Chao MP, Majeti R, Weissman IL . Macrophages as mediators of tumor immunosurveillance. Trends Immunol 2010; 31: 212–219.
Liu X, Pu Y, Cron K, Deng L, Kline J, Frazier WA et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med 2015; 21: 1209–1215.
Ame-Thomas P, Tarte K . The yin and the yang of follicular lymphoma cell niches: role of microenvironment heterogeneity and plasticity. Semin Cancer Biol 2014; 24: 23–32.
Burger JA, Tsukada N, Burger M, Zvaifler NJ, Dell'Aquila M, Kipps TJ . Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood 2000; 96: 2655–2663.
Burkle A, Niedermeier M, Schmitt-Graff A, Wierda WG, Keating MJ, Burger JA . Overexpression of the CXCR5 chemokine receptor, and its ligand, CXCL13 in B-cell chronic lymphocytic leukemia. Blood 2007; 110: 3316–3325.
Tsukada N, Burger JA, Zvaifler NJ, Kipps TJ . Distinctive features of "nurselike" cells that differentiate in the context of chronic lymphocytic leukemia. Blood 2002; 99: 1030–1037.
Polk A, Lu Y, Wang T, Seymour E, Bailey NG, Singer JW et al. Colony-stimulating factor-1 receptor is required for nurse-like cell survival in chronic lymphocytic leukemia. Clin Cancer Res 2016; e-pub ahead of print 22 June 2016.
Deaglio S, Vaisitti T, Bergui L, Bonello L, Horenstein AL, Tamagnone L et al. CD38 and CD100 lead a network of surface receptors relaying positive signals for B-CLL growth and survival. Blood 2005; 105: 3042–3050.
Tonino SH, Spijker R, Luijks DM, van Oers MH, Kater AP . No convincing evidence for a role of CD31-CD38 interactions in the pathogenesis of chronic lymphocytic leukemia. Blood 2008; 112: 840–843.
Filip AA, Cisel B, Koczkodaj D, Wasik-Szczepanek E, Piersiak T, Dmoszynska A . Circulating microenvironment of CLL: are nurse-like cells related to tumor-associated macrophages? Blood Cells Mol Dis 2013; 50: 263–270.
Audrito V, Serra S, Brusa D, Mazzola F, Arruga F, Vaisitti T et al. Extracellular nicotinamide phosphoribosyltransferase (NAMPT) promotes M2 macrophage polarization in chronic lymphocytic leukemia. Blood 2015; 125: 111–123.
Gautam S, Fatehchand K, Elavazhagan S, Reader BF, Ren L, Mo X et al. Reprogramming nurse-like cells with interferon gamma to interrupt chronic lymphocytic leukemia cell survival. J Biol Chem 2016; 291: 14356–14362.
Seiffert M, Schulz A, Ohl S, Dohner H, Stilgenbauer S, Lichter P . Soluble CD14 is a novel monocyte-derived survival factor for chronic lymphocytic leukemia cells, which is induced by CLL cells in vitro and present at abnormally high levels in vivo. Blood 2010; 116: 4223–4230.
Jia L, Clear A, Liu FT, Matthews J, Uddin N, McCarthy A et al. Extracellular HMGB1 promotes differentiation of nurse-like cells in chronic lymphocytic leukemia. Blood 2014; 123: 1709–1719.
Mosser DM, Edwards JP . Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8: 958–969.
Schulz A, Toedt G, Zenz T, Stilgenbauer S, Lichter P, Seiffert M . Inflammatory cytokines and signaling pathways are associated with survival of primary chronic lymphocytic leukemia cells in vitro: a dominant role of CCL2. Haematologica 2011; 96: 408–416.
Hanna BS, McClanahan F, Yazdanparast H, Zaborsky N, Kalter V, Rossner PM et al. Depletion of CLL-associated patrolling monocytes and macrophages controls disease development and repairs immune dysfunction in vivo. Leukemia 2016; 30: 570–579.
Nishio M, Endo T, Tsukada N, Ohata J, Kitada S, Reed JC et al. Nurselike cells express BAFF and APRIL, which can promote survival of chronic lymphocytic leukemia cells via a paracrine pathway distinct from that of SDF-1alpha. Blood 2005; 106: 1012–1020.
Ghamlouch H, Ouled-Haddou H, Damaj G, Royer B, Gubler B, Marolleau JP . A combination of cytokines rescues highly purified leukemic CLL B-cells from spontaneous apoptosis in vitro. PLoS One 2013; 8: e60370.
Bojarska-Junak A, Hus I, Chocholska S, Wasik-Szczepanek E, Sieklucka M, Dmoszynska A et al. BAFF and APRIL expression in B-cell chronic lymphocytic leukemia: correlation with biological and clinical features. Leuk Res 2009; 33: 1319–1327.
Enzler T, Kater AP, Zhang W, Widhopf GF 2nd, Chuang HY, Lee J et al. Chronic lymphocytic leukemia of Emu-TCL1 transgenic mice undergoes rapid cell turnover that can be offset by extrinsic CD257 to accelerate disease progression. Blood 2009; 114: 4469–4476.
Lascano V, Guadagnoli M, Schot JG, Luijks DM, Guikema JE, Cameron K et al. Chronic lymphocytic leukemia disease progression is accelerated by APRIL-TACI interaction in the TCL1 transgenic mouse model. Blood 2013; 122: 3960–3963.
Haderk F, Hanna B, Richter K, Schnolzer M, Zenz T, Stilgenbauer S et al. Extracellular vesicles in chronic lymphocytic leukemia. Leuk Lymphoma 2013; 54: 1826–1830.
Bichi R, Shinton SA, Martin ES, Koval A, Calin GA, Cesari R et al. Human chronic lymphocytic leukemia modeled in mouse by targeted TCL1 expression. Proc Natl Acad Sci USA 2002; 99: 6955–6960.
Reinart N, Nguyen PH, Boucas J, Rosen N, Kvasnicka HM, Heukamp L et al. Delayed development of chronic lymphocytic leukemia in the absence of macrophage migration inhibitory factor. Blood 2013; 121: 812–821.
Binsky-Ehrenreich I, Marom A, Sobotta MC, Shvidel L, Berrebi A, Hazan-Halevy I et al. CD84 is a survival receptor for CLL cells. Oncogene 2014; 33: 1006–1016.
Galletti G, Scielzo C, Barbaglio F, Rodriguez TV, Riba M, Lazarevic D et al. Targeting macrophages sensitizes chronic lymphocytic leukemia to apoptosis and inhibits disease progression. Cell Rep 2016; 14: 1748–1760.
Binder M, Lechenne B, Ummanni R, Scharf C, Balabanov S, Trusch M et al. Stereotypical chronic lymphocytic leukemia B-cell receptors recognize survival promoting antigens on stromal cells. PLoS One 2010; 5: e15992.
Hume DA, MacDonald KP . Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1 R) signaling. Blood 2012; 119: 1810–1820.
MacDonald KP, Palmer JS, Cronau S, Seppanen E, Olver S, Raffelt NC et al. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood 2010; 116: 3955–3963.
Cannarile MA, Ries CH, Hoves S, Ruttinger D . Targeting tumor-associated macrophages in cancer therapy and understanding their complexity. Oncoimmunology 2014; 3: e955356.
Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V et al. Targeting tumor-associated macrophages with anti-CSF-1 R antibody reveals a strategy for cancer therapy. Cancer Cell 2014; 25: 846–859.
Ries CH, Hoves S, Cannarile MA, Ruttinger D . CSF-1/CSF-1 R targeting agents in clinical development for cancer therapy. Curr Opin Pharmacol 2015; 23: 45–51.
Cassier PA, Italiano A, Gomez-Roca CA, Le Tourneau C, Toulmonde M, Cannarile MA et al. CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: a dose-escalation and dose-expansion phase 1 study. Lancet Oncol 2015; 16: 949–956.
Skarzynski M, Niemann CU, Lee YS, Martyr S, Maric I, Salem D et al. Interactions between ibrutinib and anti-CD20 antibodies: competing effects on the outcome of combination therapy. Clin Cancer Res 2016; 22: 86–95.
Niemann CU, Herman SE, Maric I, Gomez-Rodriguez J, Biancotto A, Chang BY et al. Disruption of in vivo chronic lymphocytic leukemia tumor-microenvironment interactions by ibrutinib - findings from an investigator-initiated phase II study. Clin Cancer Res 2016; 22: 1572–1582.
Germano G, Frapolli R, Belgiovine C, Anselmo A, Pesce S, Liguori M et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell 2013; 23: 249–262.
Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD Jr et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 2009; 138: 286–299.
Weiskopf K, Weissman IL . Macrophages are critical effectors of antibody therapies for cancer. MAbs 2015; 7: 303–310.
Kim D, Wang J, Willingham SB, Martin R, Wernig G, Weissman IL . Anti-CD47 antibodies promote phagocytosis and inhibit the growth of human myeloma cells. Leukemia 2012; 26: 2538–2545.
Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 2010; 142: 699–713.
Chanan-Khan AA, Cheson BD . Lenalidomide for the treatment of B-cell malignancies. J Clin Oncol 2008; 26: 1544–1552.
Wendtner CM, Hillmen P, Mahadevan D, Buhler A, Uharek L, Coutre S et al. Final results of a multicenter phase 1 study of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia. Leuk Lymphoma 2012; 53: 417–423.
Gorgun G, Ramsay AG, Holderried TA, Zahrieh D, Le Dieu R, Liu F et al. E(mu)-TCL1 mice represent a model for immunotherapeutic reversal of chronic lymphocytic leukemia-induced T-cell dysfunction. Proc Natl Acad Sci USA 2009; 106: 6250–6255.
Schulz A, Durr C, Zenz T, Dohner H, Stilgenbauer S, Lichter P et al. Lenalidomide reduces survival of chronic lymphocytic leukemia cells in primary cocultures by altering the myeloid microenvironment. Blood 2013; 121: 2503–2511.
Acknowledgements
This project was supported by CLL Global Research Foundation and by Associazione Italiana per la Ricerca sul Cancro (AIRC; Program Molecular Clinical Oncology-5 per mille number 9965), Milan, Italy. Figures were obtained using Servier Medical Art: www.servier.com.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
MTSB has received research funding from Roche and Celgene. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Galletti, G., Caligaris-Cappio, F. & Bertilaccio, M. B cells and macrophages pursue a common path toward the development and progression of chronic lymphocytic leukemia. Leukemia 30, 2293–2301 (2016). https://doi.org/10.1038/leu.2016.261
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2016.261
This article is cited by
-
Chronic lymphocytic leukemia in 2020: a surfeit of riches?
Leukemia (2020)
-
Specific NOTCH1 antibody targets DLL4-induced proliferation, migration, and angiogenesis in NOTCH1-mutated CLL cells
Oncogene (2020)
-
Revisiting the role of interleukin-8 in chronic lymphocytic leukemia
Scientific Reports (2017)