Abstract
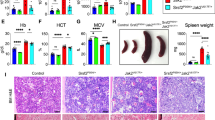
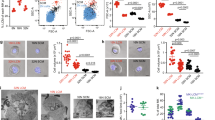
The myeloproliferative neoplasms (MPNs) are characterized by hematopoietic stem/progenitor cell (HSPC) expansion and overproduction of mature blood cells. The JAK2V617F mutation is present in hematopoietic cells in a majority of patients with MPNs, but the mechanism(s) responsible for MPN stem cell expansion remain incomplete. One hallmark feature of the marrow in patients with MPNs is megakaryocyte (MK) hyperplasia. We report here that mice bearing a human JAK2V617F gene restricted exclusively to the MK lineage develop many of the features of a MPN. Specifically, these mice exhibit thrombocytosis, splenomegaly, increased numbers of marrow and splenic hematopoietic progenitors and a substantial expansion of HSPCs. In addition, wild-type mice transplanted with cells from JAK2V617F-bearing MK marrow develop a myeloproliferative syndrome with thrombocytosis and erythrocytosis as well as pan-hematopoietic progenitor and stem cell expansion. As marrow histology in this murine model of myeloproliferation reveals a preferentially perivascular localization of JAK2V617F-mutant MKs and an increased marrow sinusoid vascular density, it adds to accumulating data that MKs are an important component of the marrow HSPC niche, and that MK expansion might indirectly contribute to the critical role of the thrombopoietin/c-Mpl signaling pathway in HSPC maintenance and expansion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003; 425: 841–846.
Zhang J, Niu C, Ye L, Huang H, He X, Tong WG et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 2003; 425: 836–841.
Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 2004; 118: 149–161.
Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ . SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 2005; 121: 1109–1121.
Morrison SJ, Scadden DT . The bone marrow niche for haematopoietic stem cells. Nature 2014; 505: 327–334.
Sipkins DA, Wei X, Wu JW, Runnels JM, Cote D, Means TK et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature 2005; 435: 969–973.
Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 2013; 502: 637–643.
Tamplin OJ, Durand EM, Carr LA, Childs SJ, Hagedorn EJ, Li P et al. Hematopoietic stem cell arrival triggers dynamic remodeling of the perivascular niche. Cell 2015; 160: 241–252.
Yin T, Li L . The stem cell niches in bone. J Clin Invest 2006; 116: 1195–1201.
Walkley CR, Olsen GH, Dworkin S, Fabb SA, Swann J, McArthur GA et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell 2007; 129: 1097–1110.
Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature 2010; 464: 852–857.
Geyh S, Oz S, Cadeddu RP, Frobel J, Bruckner B, Kundgen A et al. Insufficient stromal support in MDS results from molecular and functional deficits of mesenchymal stromal cells. Leukemia 2013; 27: 1841–1851.
Zhang B, Ho YW, Huang Q, Maeda T, Lin A, Lee SU et al. Altered microenvironmental regulation of leukemic and normal stem cells in chronic myelogenous leukemia. Cancer Cell 2012; 21: 577–592.
Schepers K, Pietras EM, Reynaud D, Flach J, Binnewies M, Garg T et al. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell Stem Cell 2013; 13: 285–299.
Ludin A, Itkin T, Gur-Cohen S, Mildner A, Shezen E, Golan K et al. Monocytes-macrophages that express alpha-smooth muscle actin preserve primitive hematopoietic cells in the bone marrow. Nat Immunol 2012; 13: 1072–1082.
Chow A, Lucas D, Hidalgo A, Mendez-Ferrer S, Hashimoto D, Scheiermann C et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med 2011; 208: 261–271.
Li JY, Adams J, Calvi LM, Lane TF, DiPaolo R, Weitzmann MN et al. PTH expands short-term murine hemopoietic stem cells through T cells. Blood 2012; 120: 4352–4362.
Junt T, Schulze H, Chen Z, Massberg S, Goerge T, Krueger A et al. Dynamic visualization of thrombopoiesis within bone marrow. Science 2007; 317: 1767–1770.
Zhao M, Perry JM, Marshall H, Venkatraman A, Qian P, He XC et al. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med 2014; 20: 1321–1326.
Bruns I, Lucas D, Pinho S, Ahmed J, Lambert MP, Kunisaki Y et al. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med 2014; 20: 1315–1320.
Malara A, Currao M, Gruppi C, Celesti G, Viarengo G, Buracchi C et al. Megakaryocytes contribute to the bone marrow-matrix environment by expressing fibronectin, type IV collagen, and laminin. Stem Cells 2014; 32: 926–937.
Zhao M, Ross JT, Itkin T, Perry JM, Venkatraman A, Haug JS et al. FGF signaling facilitates postinjury recovery of mouse hematopoietic system. Blood 2012; 120: 1831–1842.
Heazlewood SY, Neaves RJ, Williams B, Haylock DN, Adams TE, Nilsson SK . Megakaryocytes co-localise with hemopoietic stem cells and release cytokines that up-regulate stem cell proliferation. Stem Cell Res 2013; 11: 782–792.
Arranz L, Sanchez-Aguilera A, Martin-Perez D, Isern J, Langa X, Tzankov A et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature 2014; 512: 78–81.
Mager LF, Riether C, Schurch CM, Banz Y, Wasmer MH, Stuber R et al. IL-33 signaling contributes to the pathogenesis of myeloproliferative neoplasms. J Clin Invest 2015; 125: 2579–2591.
Rondelli D, Goldberg JD, Isola L, Price LS, Shore TB, Boyer M et al. MPD-RC 101 prospective study of reduced-intensity allogeneic hematopoietic stem cell transplantation in patients with myelofibrosis. Blood 2014; 124: 1183–1191.
Ciurea SO, Merchant D, Mahmud N, Ishii T, Zhao Y, Hu W et al. Pivotal contributions of megakaryocytes to the biology of idiopathic myelofibrosis. Blood 2007; 110: 986–993.
Tiedt R, Schomber T, Hao-Shen H, Skoda RC . Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood 2007; 109: 1503–1506.
Hitchcock IS, Fox NE, Prevost N, Sear K, Shattil SJ, Kaushansky K . Roles of focal adhesion kinase (FAK) in megakaryopoiesis and platelet function: studies using a megakaryocyte lineage specific FAK knockout. Blood 2008; 111: 596–604.
Ng AP, Kauppi M, Metcalf D, Hyland CD, Josefsson EC, Lebois M et al. Mpl expression on megakaryocytes and platelets is dispensable for thrombopoiesis but essential to prevent myeloproliferation. Proc Natl Acad Sci USA 2014; 111: 5884–5889.
Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 2010; 13: 133–140.
Etheridge SL, Roh ME, Cosgrove ME, Sangkhae V, Fox NE, Chen J et al. JAK2V617F-positive endothelial cells contribute to clotting abnormalities in myeloproliferative neoplasms. Proc Natl Acad Sci USA 2014; 111: 2295–2300.
Fang S, Wei J, Pentinmikko N, Leinonen H, Salven P . Generation of functional blood vessels from a single c-kit+ adult vascular endothelial stem cell. PLoS Biol 2012; 10: e1001407.
van Beijnum JR, Rousch M, Castermans K, van der Linden E, Griffioen AW . Isolation of endothelial cells from fresh tissues. Nat Protoc 2008; 3: 1085–1091.
Chagraoui H, Kassouf M, Banerjee S, Goardon N, Clark K, Atzberger A et al. SCL-mediated regulation of the cell-cycle regulator p21 is critical for murine megakaryopoiesis. Blood 2011; 118: 723–735.
Calaminus SD, Guitart AV, Sinclair A, Schachtner H, Watson SP, Holyoake TL et al. Lineage tracing of Pf4-Cre marks hematopoietic stem cells and their progeny. PloS One 2012; 7: e51361.
Tiedt R, Hao-Shen H, Sobas MA, Looser R, Dirnhofer S, Schwaller J et al. Ratio of mutant JAK2-V617F to wild-type Jak2 determines the MPD phenotypes in transgenic mice. Blood 2008; 111: 3931–3940.
Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med 2004; 10: 64–71.
Hamada T, Mohle R, Hesselgesser J, Hoxie J, Nachman RL, Moore MA et al. Transendothelial migration of megakaryocytes in response to stromal cell-derived factor 1 (SDF-1) enhances platelet formation. J Exp Med 1998; 188: 539–548.
Rafii S, Shapiro F, Pettengell R, Ferris B, Nachman RL, Moore MA et al. Human bone marrow microvascular endothelial cells support long-term proliferation and differentiation of myeloid and megakaryocytic progenitors. Blood 1995; 86: 3353–3363.
Kong Y, Hu Y, Zhang XH, Wang YZ, Mo XD, Zhang YY et al. Association between an impaired bone marrow vascular microenvironment and prolonged isolated thrombocytopenia after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2014; 20: 1190–1197.
Acar M, Kocherlakota KS, Murphy MM, Peyer JG, Oguro H, Inra CN et al. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature 2015; 526: 126–130.
Medinger M, Skoda R, Gratwohl A, Theocharides A, Buser A, Heim D et al. Angiogenesis and vascular endothelial growth factor-/receptor expression in myeloproliferative neoplasms: correlation with clinical parameters and JAK2-V617F mutational status. Br J Haematol 2009; 146: 150–157.
Boveri E, Passamonti F, Rumi E, Pietra D, Elena C, Arcaini L et al. Bone marrow microvessel density in chronic myeloproliferative disorders: a study of 115 patients with clinicopathological and molecular correlations. Br J Haematol 2008; 140: 162–168.
Gianelli U, Vener C, Raviele PR, Savi F, Somalvico F, Calori R et al. VEGF expression correlates with microvessel density in Philadelphia chromosome-negative chronic myeloproliferative disorders. Am J Clin Pathol 2007; 128: 966–973.
Malara A, Abbonante V, Di Buduo CA, Tozzi L, Currao M, Balduini A . The secret life of a megakaryocyte: emerging roles in bone marrow homeostasis control. Cell Mol Life Sci 2015; 72: 1517–1536.
deHaan G, Weersing E, Dontje B, van Os R, Bystrykh LV, Vellenga E et al. In vitro generation of long-term repopulating hematopoietic stem cells by fibroblast growth factor-1. Dev Cell 2003; 4: 241–251.
Kaushansky K, Lok S, Holly RD, Broudy VC, Lin N, Bailey MC et al. Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature 1994; 369: 568–571.
Sitnicka E, Lin N, Priestley GV, Fox N, Broudy VC, Wolf NS et al. The effect of thrombopoietin on the proliferation and differentiation of murine hematopoietic stem cells. Blood 1996; 87: 4998–5005.
Kimura S, Roberts AW, Metcalf D, Alexander WS . Hematopoietic stem cell deficiencies in mice lacking c-Mpl, the receptor for thrombopoietin. Proc Natl Acad Sci USA 1998; 95: 1195–1200.
Solar GP, Kerr WG, Zeigler FC, Hess D, Donahue C, de Sauvage FJ et al. Role of c-mpl in early hematopoiesis. Blood 1998; 92: 4–10.
Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell 2007; 1: 685–697.
Qian H, Buza-Vidas N, Hyland CD, Jensen CT, Antonchuk J, Mansson R et al. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell 2007; 1: 671–684.
Sangkhae V, Etheridge SL, Kaushansky K, Hitchcock IS . The thrombopoietin receptor, MPL, is critical for development of a JAK2V617F-induced myeloproliferative neoplasm. Blood 2014; 124: 3956–3963.
Dominici M, Rasini V, Bussolari R, Chen X, Hofmann TJ, Spano C et al. Restoration and reversible expansion of the osteoblastic hematopoietic stem cell niche after marrow radioablation. Blood 2009; 114: 2333–2343.
Olson TS, Caselli A, Otsuru S, Hofmann TJ, Williams R, Paolucci P et al. Megakaryocytes promote murine osteoblastic HSC niche expansion and stem cell engraftment after radioablative conditioning. Blood 2013; 121: 5238–5249.
Mullally A, Lane SW, Ball B, Megerdichian C, Okabe R, Al-Shahrour F et al. Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer cell 2010; 17: 584–596.
Xing S, Wanting TH, Zhao W, Ma J, Wang S, Xu X et al. Transgenic expression of JAK2V617F causes myeloproliferative disorders in mice. Blood 2008; 111: 5109–5117.
Akada H, Yan D, Zou H, Fiering S, Hutchison RE, Mohi MG . Conditional expression of heterozygous or homozygous Jak2V617F from its endogenous promoter induces a polycythemia vera-like disease. Blood 2010; 115: 3589–3597.
Li J, Spensberger D, Ahn JS, Anand S, Beer PA, Ghevaert C et al. JAK2 V617F impairs hematopoietic stem cell function in a conditional knock-in mouse model of JAK2 V617F-positive essential thrombocythemia. Blood 2010; 116: 1528–1538.
Acknowledgements
The authors thank Dr Ian Hitchcock (University of York, UK) for his scientific consultation and Ms. Laurie Levine (Stony Brook University, NY, USA) for her assistance with the animal work. We thank Dr Radek Skoda (University Hospital Basal, Switzerland) for furnishing the mice (FF1 and Pf4-Cre) and for many fruitful discussions. We thank Drs Stanley Zucker and Hussein Foda(Northport VA Medical Center) for their continuing support throughout this work. This research was supported by the Veterans Affairs Career Development Award CDA210959632 (HZ) and National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK049855 (KK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Zhan, H., Ma, Y., Lin, C. et al. JAK2V617F-mutant megakaryocytes contribute to hematopoietic stem/progenitor cell expansion in a model of murine myeloproliferation. Leukemia 30, 2332–2341 (2016). https://doi.org/10.1038/leu.2016.114
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2016.114
This article is cited by
-
JAK2 inhibitor persistence in MPN: uncovering a central role of ERK activation
Blood Cancer Journal (2022)
-
The Rationale for Immunotherapy in Myeloproliferative Neoplasms
Current Hematologic Malignancy Reports (2019)
-
The JAK2V617F-bearing vascular niche promotes clonal expansion in myeloproliferative neoplasms
Leukemia (2018)